| Company Name: |
3B Pharmachem (Wuhan) International Co.,Ltd.
|
| Tel: |
821-50328103-801 18930552037 |
| Email: |
3bsc@sina.com |
| Products Intro: |
Product Name:GR 113808;1-Methyl-1H-indole-3-carboxylicacid,[1-[2-[(Methylsulfonyl)aMino]ethyl]-4-piperidinyl]Methylester
CAS:144625-51-4
Purity:99% HPLC Package:1Mg ; 5Mg;10Mg ;100Mg;250Mg ;500Mg ;1g;2.5g ;5g ;10g
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Email: |
orderCN@merckgroup.com |
| Products Intro: |
Product Name:GR 113808
CAS:144625-51-4
Purity:>=98% (HPLC), solid Package:50MG Remarks:G5918-50MG
|
|
| | GR 113808 Basic information |
| Product Name: | GR 113808 | | Synonyms: | 1-METHYL-1H-INDOLE-3-CARBOXYLIC ACID, [1-[2-[(METHYLSULFONYL)AMINO]ETHYL]-4-PIPERIDINYL]METHYL ESTER;1-[2-(METHYLSULFONYL)-AMINO]ETHYL-4-PIPERIDINYLMETHYL 1-METHYL-1H-INDOLE-3-CARBOXYLATE;GR 113808;1-METHYL-1H-INDOLE-3-CARBOXYLIC ACID, [1-[2-[(METHYLSULFONYL)AMINO]ETHYL]-4-PIPERIDINYL]METHYL ESTER;1-[2-(METHYLSULFONYL)-AMINO]ETHYL-4-PIPERIDINYLMETHYL 1-METHYL-1H-INDOLE-3-CARBOXYLATE;GR 113808;1-Methyl-1H-indole-3-carboxylicacid,[1-[2-[(methylsulfonyl)amino]ethyl]-4-piperidinyl]methyles;GR 113808;1H-Indole-3-carboxylic acid, 1-methyl-, [1-[2-[(methylsulfonyl)amino]ethyl]-4-piperidinyl]methyl ester;GR113808, selective 5-HT4 antagonist | | CAS: | 144625-51-4 | | MF: | C19H27N3O4S | | MW: | 393.5 | | EINECS: | | | Product Categories: | Serotonin receptor | | Mol File: | 144625-51-4.mol | 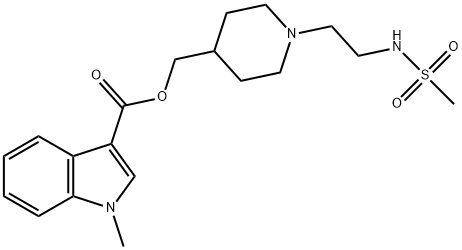 |
| | GR 113808 Chemical Properties |
| Boiling point | 572.3±53.0 °C(Predicted) | | density | 1.30±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO: 44 mg/mL, soluble | | pka | 11.22±0.40(Predicted) | | form | solid | | color | white | | InChI | 1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | | InChIKey | MOZPSIXKYJUTKI-UHFFFAOYSA-N |
| WGK Germany | 3 | | Storage Class | 11 - Combustible Solids |
| | GR 113808 Usage And Synthesis |
| Uses | GR 113808 has been used as a serotonin receptor 4 (5-HT4R) blocker to study its effects on the calcium (Ca2+) transients in rat ventricular cardiomyocytes. It has also been used as a 5-HT4R antagonist to study its effects on de novo enteric neurogenesis in post-embryonic zebrafish. | | Definition | ChEBI: An indolyl carboxylate ester obtained by formal condensation between the carboxy group of 1-methylindole-3-carboxylic acid with the hydroxy group of N-{2-[4-(hydroxymethyl)piperidin-1-yl]ethyl}methanesulfonamide. | | Biological Activity | Potent, selective 5-HT 4 receptor antagonist (pK B = 9.43 in human colonic muscle, and K d = 0.15 nM for binding to cloned human 5-HT 4 receptors). Displays > 300-fold selectivity over 5-HT 1A , 5-HT 1B , 5-HT 2A , 5-HT 2C and 5-HT 3 receptors. | | Biochem/physiol Actions | GR 113808 is a 5-HT4 serotonin receptor antagonist. | | storage | Store at RT |
| | GR 113808 Preparation Products And Raw materials |
|