- TAPSO
-

- $0.00 / 1kg
-
2026-01-15
- CAS:68399-81-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- TAPSO
-

- $0.00 / 1KG
-
2026-01-15
- CAS:68399-81-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 3500kg/month
- Tapso
-

- $30.00 / 1KG
-
2025-06-27
- CAS:68399-81-5
- Min. Order: 50KG
- Purity: 99%
- Supply Ability: 500000kg
|
| Product Name: | TAPSO | | Synonyms: | 3-[N-Tris-(hydroxyMethyl)MethylaMino]-2-hydroxypropanesulphonic acid(TAPSO);TAPSO(2-Hydroxy-3-[tris(hydroxyMethylaMino]-1 propanesulfonic acid);TAPSO Vetec(TM) reagent grade, >=99%;N-[Tris(hydroxymethyl)metyl]-3-amino-2-hydroxypropanesulfonic acid;Tapso≥ 99.9% (titration);TAPSO, 99+%, FOR BIOCHEMISTRY;TAPSO, for biochemistry;2-Hydroxy-3-[[1,1-bis(hydroxymethyl)-2-hydroxyethyl]amino]-1-propanesulfonic acid | | CAS: | 68399-81-5 | | MF: | C7H17NO7S | | MW: | 259.28 | | EINECS: | 269-993-8 | | Product Categories: | Buffer;Biochemistry;Good's Buffers | | Mol File: | 68399-81-5.mol | 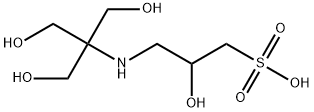 |
| | TAPSO Chemical Properties |
| Melting point | 224-229 °C (dec.) | | density | 1.07607 g/cm3(Temp: 25.00 °C) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | H2O: 1 M at 20 °C, clear, colorless | | form | Powder | | color | Off-white to yellow | | PH | 7.0-8.2 | | PH Range | 7.0 - 8.2 | | pka | 7.6(at 25℃) | | Water Solubility | soluble | | Merck | 13,9148 | | BRN | 2113949 | | Major Application | diagnostic assay manufacturing | | InChI | InChI=1S/C7H17NO7S/c9-3-7(4-10,5-11)8-1-6(12)2-16(13,14)15/h6,8-12H,1-5H2,(H,13,14,15) | | InChIKey | RZQXOGQSPBYUKH-UHFFFAOYSA-N | | SMILES | C(S(O)(=O)=O)C(O)CNC(CO)(CO)CO | | CAS DataBase Reference | 68399-81-5(CAS DataBase Reference) | | EPA Substance Registry System | 1-Propanesulfonic acid, 2-hydroxy-3-[[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino]- (68399-81-5) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | TSCA | TSCA listed | | HazardClass | IRRITANT | | HS Code | 29221990 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| | TAPSO Usage And Synthesis |
| Chemical Properties | white powder | | Uses | TAPSO is a zwitterionic biological buffer often used as a buffering agent in biological and biochemical research. It has a pKa value of 7.635 (I=0, 25°C) and can be used to make buffer solutions in the pH range 7.0-8.2. As an Good'buffer, TAPSO contains Tris groups and exhibits quite reactive activity with zwitterionic glycine peptides[1]. TAPSO can serve as good stabilizers for the globular protein BSA, in the aqueous solutions[2].The solubilities of TAPSO increase with increasing concentration of the salts (salting-in effect)[3]. | | Synthesis | N-Tris(hydroxymethyl)methane-2-hydroxypropanesulfonic acid was prepared as follows: a mixture of 242.28 g (2 mol) of tris(hydroxymethyl)aminomethane, 500 ml of water and 196.5 g (1 mol) of sodium 3-chloro-2-hydroxypropanesulphonate was charged into a 1-liter 2-necked flask fitted with a stirrer and condenser. The mixture was stirred vigorously at reflux for 3 hours. After cooling to 25??C, the solution was allowed to pass through a Dowex 50, hydrogen form cation exchange resin column to remove the sodium cation and form a free acid. The eluate from the column was evaporated under vacuum to a thick oil. After dilution with alcohol, the product was crystallized from the solution, filtered and washed with alcohol and dried. 160 g of the white product N-tris(hydroxymethyl)methionine-2-hydroxypropanesulfonic acid was obtained. | | References | [1] Gupta B, et al. Stability Constants for the Equilibrium Models of Iron(III) with Several Biological Buffers in Aqueous Solutions. Journal of Solution Chemistry, 2013; 42: 2296–2309.
[2] Gupta ;B, et al. Interactions of bovine serum albumin with biological buffers, TES, TAPS, and TAPSO in aqueous solutions. Process Biochemistry, 2013; 48: 1686-1696.
[3] Taha M, et al. New Insights into Buffer-Ionic Salt Interactions: Solubilities, Transfer Gibbs Energies, and Transfer Molar Volumes of TAPS and TAPSO from Water to Aqueous Electrolyte Solutions. Journal of Solution Chemistry, 2010; 39:1665–1680. |
| | TAPSO Preparation Products And Raw materials |
|