| Company Name: |
ChemStrong Scientific Co.,Ltd
|
| Tel: |
0755-66853366 13670046396 |
| Email: |
sales@chem-strong.com |
| Products Intro: |
Product Name:Oxacillin sodium Impurity 12
CAS:724695-30-1
Purity:95% HPLC Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Shanghai Kewel Chemical Co., Ltd.
|
| Tel: |
021-64609169 18901607656 |
| Email: |
greensnown@163.com |
| Products Intro: |
Product Name:Histamine Dihydrochloride Impurity 3
CAS:724695-30-1
Purity:95+ HPLC; Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Hubei Yangxin Medical Technology Co., Ltd.
|
| Tel: |
15374522761 |
| Email: |
3003392093@yongstandards.com |
| Products Intro: |
Product Name:Oxacillin EP Impurity G
CAS:724695-30-1
Purity:99%+ HPLC Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Shanghai CR Corporation Limited
|
| Tel: |
13062833949; 4006653949 4006653949 |
| Email: |
fred.wen@crcorporation.cn |
| Products Intro: |
Product Name:Oxacillin EP Impurity
CAS:724695-30-1
Purity:95+
|
|
| | Oxacillin Sodium Impurity G(EP) Basic information |
| Product Name: | Oxacillin Sodium Impurity G(EP) | | Synonyms: | Oxacillin Sodium Impurity G(EP);Oxacillin Impurity G;Oxacillin EP Impurity G;(2S,5R,6R)-6-(3-(3-chlorophenyl)-5-methylisoxazole-4-carboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2S,5R,6R)-6-(3-(2-chlorophenyl)-5-methylisoxazole-4-carboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with (2S,5R,6R)-6-(3-(3-chlorophenyl)-5-methylisoxazole-4-carboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;Oxacillin sodium Impurity 12;Oxacillin Impurity 21;Oxacillin Sodium Impurity G | | CAS: | 724695-30-1 | | MF: | C19H18ClN3O5S | | MW: | 435.88 | | EINECS: | | | Product Categories: | | | Mol File: | 724695-30-1.mol | 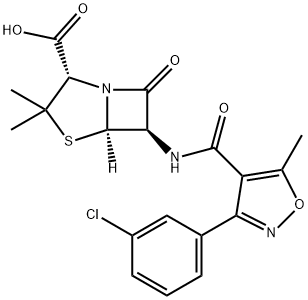 |
| | Oxacillin Sodium Impurity G(EP) Chemical Properties |
| Boiling point | 706.9±60.0 °C(Predicted) | | density | 1.56±0.1 g/cm3(Predicted) | | pka | 2.44±0.50(Predicted) |
| | Oxacillin Sodium Impurity G(EP) Usage And Synthesis |
| | Oxacillin Sodium Impurity G(EP) Preparation Products And Raw materials |
|