Application research of 9-vinylcarbazole
Oct 30,2025
Introduction
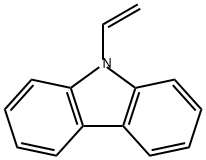
9-Vinylcarbazole (Figure 1) is a significant nitrogen-containing aromatic heterocyclic compound that exhibits unique photoelectric properties, aligning well with the performance characteristics of organic photoelectric functional materials and possessing a strong intramolecular electron transfer capability. Additionally, it boasts relatively good thermal stability, serving as a crucial intermediate for synthesizing optoelectronic materials and finding widespread application in organic optoelectronic functional materials. 9-Vinylcarbazole acts as the monomer raw material for synthesizing poly(9-vinylcarbazole) (PVK), a classic and important polymer primarily characterized by hole conductivity.[1] Herein,the application research of 9-vinylcarbazole or poly(9-vinylcarbazole) will be introduced.

Synthetic Process of 9-Vinylcarbazole
Report 1
For the first time,carbazole and methylbutynol were used as raw materials,and high- purified 9-vinylcarbazole was synthesized under KOH basic conditions. The screening optimization results were obtained as follow:The molar ratio n(carbazole)∶n(methylbutynol)∶n(KOH)=1∶1.1∶1.1,ethanol as the solvent,reaction temperature 78°C,reaction time 2.5 h. The highest yield of the product can reach 81.3% and the purity was 99.5%.The method is suitable for industrial production because of its mild reaction conditions,simple operation and high yield.[1]
Report 2
9- vinylcarbazole was produced by reaction from carbazole and methyl butanediol, and the pure 9-vinylcarbazole was obtained by further treatment of reaction liquid.The effects of reaction solvent, reaction temperature and reaction time on the yield of 9- vinylcarbazole were investigated. The results showed that the best solvent for the synthetic product was DMSO, the best reaction temperature was 150 °C, and the best reaction time was 12 h. The structure of the product was characterized by FTIR, 1H NMR and 13C NMR.The liquid chromatography purity of 9-vinylcarbazole was >99.5%, the gas chromatography purity was > 99.5%, the melting point was 64 °C, the total yield was 85 %, the synthetic products can be used as electronic grade chemicals.[2]
Application of 9-vinylcarbazole
Example1 of poly(9-vinylcarbazole) application
Metal nanoparticle (NP) incorporated conductive polymer films are attractive for their mechanical stability for biomedical applications and as heterogeneous electrocatalysis materials. Novel approaches to generate these materials with tunable properties are still being sought. Herein, the interface between two immiscible electrolyte solutions (ITIES) has been employed as a molecularly sharp and reproducible platform for simultaneous Au NP and poly(9-vinylcarbazole) generation. Three interfaces have been compared, including between water|1,2-dichloroethane (w|DCE), water|α,α,α-trifluorotoluene (w|TFT), and water|ionic liquid (w|IL). In this case the IL was P8888TB (tetraoctylphosphonium tetrakis(pentafluorophenyl)borate). 9-Vinylcarbazole (VC) can polymerize via two routes, either propagating through the vinyl substituent or the aryl rings. The former gives rise to a white semiconducting polymer with a wide bandgap, while the latter produces a green, conducting polymer. External potential control through voltammetric cycling was found to generate the film more rapidly favoring heterogeneous electron transfer with formation of the green poly(VC) variant at the ITIES. This was a free-standing film that could be easily removed from the interface. In the absence of external control, white polymer crystals formed within the oil phase spontaneously likely via AuCl4- w → o transfer followed by a homogeneous electron transfer reaction mechanism. Scanning electrochemical microscopy probe approach curve experiments were used to quantify the electroactivity of the film and are complemented by direct conductivity measurements.[3]
Example 2
In this paper, a cross-linked poly(9-vinylcarbazole) (PVK):phosphomolybdic acid (PMA) layer is used as the hole transport layer in perovskite light-emitting devices, and the morphology, crystal structure, and photophysical properties of perovskite films on the PVK:PMA layer are studied. The addition of PMA into the PVK layer improves the perovskite morphology integrity and promotes hole transport. As a result, perovskite light-emitting devices using a PVK:PMA hole transport layer exhibit an improved maximum luminous efficiency of 22.1 cd A-1 and power efficiency of 18.2 lm W-1 when compared with those of the counterparts with a PVK hole transport layer. Efficient perovskite light-emitting devices can be accessed by using various antisolvents due to the good solvent resistance of PVK:PMA networks. Moreover, the luminous efficiencies of perovskite light-emitting devices with a PVK:PMA hole transport layer are almost invariant irrespective of the presence of a hole injection layer, illustrating wide applicability of the PVK:PMA hole transport layer in perovskite light-emitting devices.[4]
Example 3
The research team has developed new plastic scintillators in the form of microspheres, called PSm, by combining styrene, 9-vinylcarbazole (VK), and 4-vinylbenzyl chloride (VBC). The primary objective of this study was to explore the feasibility of incorporating the fluorescent solute (VK) into the polymer structure to prevent its leaching out when PSm are utilized in liquid flow through detection systems or organic solvents. The secondary aim was to examine the impact of adding the chlorine functional group to the scintillation polymer, with the intention of replacing it with an extractant in the future to create covalently linked PSresins. The findings of the study reveal that the homopolymer of polyvinylcarbazole (PVK) performs poorly while used as a unitary scintillator system for plastic scintillation measurements. However, the incorporation of monomers in the form of copolymers with styrene has a more significant impact on scintillation properties compared to the mixture of homopolymers. In the case of 9-vinylcarbazole (VK), its presence at a weight proportion of 10% leads to an increase in scintillation efficiencies, although it is still inferior to the classical PS. Conversely, the situation is different for 4-vinylbenzyl chloride (VBC), where the chlorine in the copolymer results in higher quenching, and the polymer is also less resistant to organic solvents due to the formation of short polymer chains. For VBC, the mixture of polymers yields better results and enables the production of covalently linked PSresins.[5]
References
1.LIU CC, et al. New Synthetic Process of N-Vinylcarbazole[J].HeNan Science,2020,38(06):886-890.DOI:CNKI:SUN:HNKX.0.2020-06-005.
2.XU HR,et al. Synthesis and Characterization of N-vinyl Carbazole[J].Contemporary Chemical Industry,2022,51(04):800-804.DOI:10.13840/j.cnki.cn21-1457/tq.2022.04.035.
3.Nazari L, Stockmann TJ. Comparison of Au Nanoparticle/Poly(9-vinylcarbazole) Thin-Film Electrogeneration at 3 Distinct Liquid/Liquid Interfaces: Water/1,2-Dichloroethane, /α,α,α-Trifluorotoluene, Or/Ionic Liquid. Langmuir. 2024;40(46):24494-24506. doi:10.1021/acs.langmuir.4c03265
4.Wu Y, Xiao Z, He L, et al. Widely applicable phosphomolybdic acid doped poly(9-vinylcarbazole) hole transport layer for perovskite light-emitting devices. RSC Adv. 2019;9(52):30398-30405. Published 2019 Sep 25. doi:10.1039/c9ra05734j
5.Serraïma-López D, Guillén M, Bagán H, Tarancón A. Scintillation and structural properties of copolymers and mixtures of styrene, 9-vinylcarbazole and 4-vinylbenzyl chloride based plastic scintillators. Appl Radiat Isot. 2024;211:111409. doi:10.1016/j.apradiso.2024.111409
- Related articles
- Related Qustion
The Lewis structure of fluorine (C2H5F) consists of two carbon atoms (C), five hydrogen atoms (H), and one fluorine atom (F).....
Oct 29,2025Organic reagentsCuSCN is a compound which has solubility characteristics in water rather similar to those of barium sulphate. Its mainly application was introduced.....
Oct 30,2025Inorganic salts9-Vinylcarbazole
1484-13-5You may like
- Application research of 1-bromo-2-butyne
Nov 3, 2025
- Application research of 2-Phenoxyethyl acrylate
Nov 3, 2025
- Application research of D-2-phenylglycine
Oct 24, 2025
9-Vinylcarbazole manufacturers
- 9-Vinylcarbazole
-

- $35.00 / 25g
- 2025-11-03
- CAS:1484-13-5
- Min. Order: 25g
- Purity: 0.98
- Supply Ability: 100kg
- 9-Vinylcarbazole
-

- $200.00 / 1KG
- 2025-09-25
- CAS:1484-13-5
- Min. Order: 1KG
- Purity: 99%, 99.5% Sublimated
- Supply Ability: g-kg-tons, free sample is available
- 9-Vinylcarbazole
-

- $0.00 / 1KG
- 2025-06-04
- CAS:1484-13-5
- Min. Order: 1KG
- Purity: 99%+
- Supply Ability: 100000kg per Month