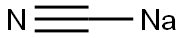사이안화 나트륨
|
|
사이안화 나트륨 속성
- 녹는점
- 563.7 °C(lit.)
- 끓는 점
- 1497°C
- 밀도
- 1.6
- 증기 밀도
- 1.7 (vs air)
- 증기압
- 1 mm Hg ( 817 °C)
- 인화점
- 1500°C
- 저장 조건
- Poison room
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 산도 계수 (pKa)
- 9.36[at 20 ℃]
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- 냄새
- The dry salts are odorless, but reaction with atmospheric moisture produces HCN, whose bitter almond odor is detectable at 1 to 5 ppm; however, 20 to 60% of the population are reported to be unable to detect the odor of HCN.
- 수소이온지수(pH)
- 11.7 (100g/l, H2O, 20°C)
- pH 범위
- 11-12
- 수용성
- 37g/100mL(20℃)
- 감도
- Hygroscopic
- Merck
- 14,8605
- BRN
- 3587243
- 노출 한도
- TLV-TWA (measured as CN) skin 5 mg CN/m3 (ACGIH and OSHA), 5 mg CN/m3/ 10-minute ceiling (NIOSH).
- Dielectric constant
- 7.6(Ambient)
- 안정성
- 흡습성
- LogP
- -0.25 at 20℃
- CAS 데이터베이스
- 143-33-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/27/28-32-50/53-48/25 | ||
| 안전지침서 | 7-28-29-45-60-61-28A | ||
| 유엔번호(UN No.) | UN 1689 6.1/PG 1 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | VZ7525000 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | I | ||
| HS 번호 | 28371110 | ||
| 유해 물질 데이터 | 143-33-9(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 15 mg/kg (Smyth) | ||
| 기존화학 물질 | KE-31401 | ||
| 유해화학물질 필터링 | 97-1-90 | ||
| 사고대비 물질 필터링 | 33 | ||
| 함량 및 규제정보 | 물질구분: 사고대비물질; 혼합물(제품)함량정보: 시안화 나트륨 및 이를 1% 이상 함유한 혼합물. 다만, 베를린청(Ferric ferrocyanide)ㆍ황혈염(Potassium ferrocyanide)ㆍ적혈염(Potassium ferri-cyanide) 및 그 중 하나를 함유한 혼합물질은 제외한다. |
사이안화 나트륨 C화학적 특성, 용도, 생산
개요
Sodium cyanide, NaCN, is a cyanide salt that is a white, deliquescent, crystalline powder and is soluble in water. The specific gravity is 1.6, which is heavier than water. Sodium cyanide is toxic by inhalation and ingestion, with a TLV of 4.7 ppm and 5 mg/m3 of air. The target organs are the cardiovascular system, central nervous system, kidneys, liver, and skin. Reactions with acids can release flammable and toxic hydrogen cyanide gas. Cyanides are incompatible with all acids. The four-digit UN identification number is 1689.The NFPA 704 designation is health 3, flammability 0, and reactivity 0. The primary uses are in gold and silver extraction from ores, electroplating, fumigation, and insecticides.
화학적 성질
Sodium cyanide is found as white granules, flakes or lumps. Sodium cyanide is shipped as pellets or briquettes. Odorless when dry. It absorbs water from air (is hygroscopic or deliquescent). Hydrogen cyanide gas released by sodium cyanide has a distinctive mild, bitter almond odor, but a large proportion of people cannot detect it; the odor does not provide adequate warning of hazardous concentrations.물리적 성질
Physical Properties White cubic crystals; hygroscopic; density 1.6 g/cm3; melts at 563°C; very soluble in water; aqueous solution strongly alkaline and decomposes rapidly.용도
Sodium cyanide is used for electroplating metals such as zinc, copper, cadmium, silver, and gold, and their alloys; for extracting gold and silver from ores; and as a fumigant and a chelating agent. It occurs in many varieties of maniocs (cassava), especially in bitter manioc.정의
sodium cyanide: A white orcolourless crystalline solid, NaCN,deliquescent, soluble in water and inliquid ammonia, and slightly solublein ethanol; cubic; m.p. 564°C; b.p.1496°C. Sodium cyanide is now madeby absorbing hydrogen cyanide insodium hydroxide or sodium carbonatesolution. The compound is extremelypoisonous because it reacts with the iron in haemoglobin in theblood, so preventing oxygen reachingthe tissues of the body. It is used inthe extraction of precious metals andin electroplating industries. Aqueoussolutions are alkaline due to salt hydrolysis.제조 방법
Sodium cyanide can be prepared by several methods (See Potassium Cyanide).It is prepared by passing hydrogen cyanide through a 50% aqueous solution of sodium hydroxide followed by evaporation of the solution in vacuum: NaOH + HCN → NaCN + H2O
Another method is to reduce sodamide with carbon at red heat: NaNH2 + C → NaCN + H2↑
Also, sodium cyanide can be made by heating a mixture of sodium carbonate and carbon with ammonia at high temperatures: Na2CO3 + 4C + 2NH3 → 2NaCN + 3CO↑ + 3H2↑.
생산 방법
Sodium cyanide was first prepared in 1834 by heating Prussian Blue, a mixture of cyanogen compounds of iron, and sodium carbonate and extracting sodium cyanide from the cooled mixture using alcohol. Sodium cyanide remained a laboratory curiosity until 1887, when a process was patented for the extraction of gold and silver ores by means of a dilute solution of cyanide.화학 반응
Sodium cyanide, NaCN, white solid, soluble, very poisonous, formed (1) by reaction of sodamide and carbon at high temperature, (2) by reaction of calcium cyanamide and sodium chloride at high temperature, reacts in dilute solution in air with gold or silver to form soluble sodium gold or silver cyanide, and used for this purpose in the cyanide process for recovery of gold. The percentage of available cyanide is greater than in potassium cyanide previously used. Used as a source of cyanide, and for hydrocyanic acid.일반 설명
A clear colorless aqueous solution.공기와 물의 반응
Slowly evolves flammable and poisonous hydrogen cyanide gas.반응 프로필
Sodium cyanide is weakly basic. Reacts with acids of all kinds to generate quantities of very poisonous hydrogen cyanide gas. Incompatible with strong oxidizing agents, especially if solution dries out. Gives insoluble products with silver(I), mercury(I) and lead(II) ions that may decompose violently under certain conditions.위험도
Toxic by ingestion and inhalation.건강위험
Sodium cyanide is a white crystalline solid that is odorless when dry, but emits a slight odor of hydrogen cyanide in damp air. It is slightly soluble in ethanol and formamide. It is very poisonous. It explodes if melted with nitrite or chlorate at about 450°F. It produces a violent reaction with magnesium, nitrites, nitrates, and nitric acid. On contact with acid, acid fumes, water, or steam, it produces toxic and flammable vapors. Synonyms for sodium cyanide are hydrocyanic acid, sodium salt, and cyanide of sodium.인화성 및 폭발성
Sodium cyanide and potassium cyanide are noncombustible solids. Reaction with acids liberates flammable HCN.공업 용도
sodium cyanide and other water-soluble cyanides are used as modifying reagents for selective flotation of ores containing galena, sphalerite and gangue minerals.잠재적 노출
Sodium cyanide is used as a solid or in solution to extract metal ores, in electroplating and metal cleaning baths; in metal hardening; in treatment of rabbit and rat burrows and holes and termite nests; in insecticides저장
In particular, work with cyanides should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Cyanide salts should be stored in a cool, dry location, separated from acids.운송 방법
UN1689 Sodium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.비 호환성
Sodium cyanide decomposes on contact with acids, acid salts, water, moisture, alcohols, and carbon dioxide, releasing highly toxic and flammable hydrogen cyanide gas. Aqueous solution is a strong base; it reacts violently with acid and is corrosive. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Absorbs moisture from the air forming a corrosive syrup. Corrosive to active metals, such as aluminum, copper, and zinc. Under acid conditions, sarin hydrolyzes to form hydrofluoric acid.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water.사이안화 나트륨 준비 용품 및 원자재
원자재
Black cyanide
Organic sulfur
Washing tower
Generator
산화납(II)
나트륨
Petroleum coke
Concentrated sulfuric acid
시안화수소
아크릴로나이트릴
Desulfurization
암모니아(가스)
준비 용품
DL-만델산
3-Pyridylacetic acid
2-(4-chlorophenyl)-3-oxovaleronitrile
Tetrahydropyranyl-4-acetic acid
2-Amino-2-phenylacetic acid
디에틸아미노아세토니트릴
1-ETHYL-1-METHYLSUCCINIC ACID
TETRAHYDRO-2H-PYRAN-4-YLACETYL CHLORIDE
5-(4-METHOXYPHENYL)HYDATOIN
Amino-4-methoxyben-zeneacetic acid
3,5-DIMETHOXYPHENYLACETONITRILE
알파시페르메트린
DL-4-HYDROXYPHENYLGLYCINE
L-Phenylglycine
브로모벤질시아나이드
DL-2-METHYLGLUTAMIC ACID
아연 시아니드
D-펜토락톤
Methyl 2-chlorophenylacetate
Benzoin
1-Naphthylacetonitrile
C-(1H-INDOL-5-YL)-METHYLAMINE
1-CYANOCYCLOPENTENE
디에틸렌트리아민펜타아세트산
5-(2-Methylthioethyl)hydantoin
2-METHOXY-5-(2'-ETHYLHEXYLOXY)BENZENE-1&
3-CHLORO-2-METHYLBENZONITRILE
2-AMINOPHENYLACETIC ACID
시할로트린
METHYL ALPHA-BROMO-2-CHLOROPHENYLACETATE
DL-만딜오리트릴
3-Methyl-2-(3,4-dimethoxyphenyl)butyronitrile
테트라메틸 티우람 황화물
Anilinoacetic acid
메틸벤질레이트
코바마마이드
METHYL 4-CYANO-3-CYCLOHEXECARBOXYLATE
2,2'아조디-2-메틸부티로니트릴
아조비스(시아노길초산)
2-Methylsuccinic acid