| Identification | More | [Name]
Ethyl N-cyanoethanimideate | [CAS]
1558-82-3 | [Synonyms]
ETHYLN-CYANOACETOIMIDATE
ETHYL N-CYANO ETHANIMIDATE
Ethyl N-cyanoethanimideate
n-cyanoethanimidic acid ethyl ester
N-CYANOETHANIMIDIC ETHYL ESTER
CYANO ETHYL ACETAMIDATE | [Molecular Formula]
C5H8N2O | [MDL Number]
MFCD03411888 | [Molecular Weight]
112.13 | [MOL File]
1558-82-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
78-81 °C(Solv: ethyl ether (60-29-7)) | [Boiling point ]
133.2±23.0 °C(Predicted) | [density ]
0.94 | [refractive index ]
1.45 | [Fp ]
34° | [storage temp. ]
2-8°C | [pka]
-2.94±0.50(Predicted) | [Appearance]
Colorless to light yellow Liquid | [InChI]
InChI=1S/C5H8N2O/c1-3-8-5(2)7-4-6/h3H2,1-2H3 | [InChIKey]
PLVWUINOWYHRAA-UHFFFAOYSA-N | [SMILES]
C(=NC#N)(OCC)C | [CAS DataBase Reference]
1558-82-3(CAS DataBase Reference) |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
To a flask equipped as in Preparation 2-6 is added 162.0 gm (1.0 mole) of ethyl orthoacetate, 42.0 gm (1.0 mole) of cyanamide, and 134.0 gm (2.0 moles) of acetic anhydride. The reaction mixture is heated to 130-140°C and the ethyl acetate and acetic acid are distilled over. The heat is removed until the initial vigorous reaction subsides and then the heating is continued at 135-140°C until most of the remaining ethyl acetate and acetid acid have distilled over (approx. 1 hr). The residue is then distilled under reduced pressure to afford 100.8 gm (90%), b.p. 90-95°C (20 mm Hg).
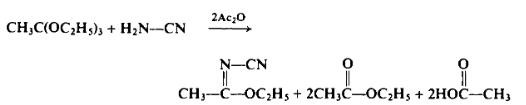
|
| Hazard Information | Back Directory | [Synthesis]
General methodology: The compound was synthesized with reference to literature methods. The standard procedure we used is detailed below: the mixed system of the original triethyl acetate (0.12 mol), cyanamide (0.1 mol) and a small amount of acetic acid (a few drops) was refluxed for 6 hours, followed by distillation and purification under reduced pressure (14 mmHg). | [References]
[1] Phosphorus, Sulfur and Silicon and the Related Elements, 2016, vol. 191, # 5, p. 759 - 764
[2] Patent: US5750545, 1998, A
[3] Patent: WO2008/42928, 2008, A2. Location in patent: Page/Page column 33
[4] Patent: WO2008/42928, 2008, A2. Location in patent: Page/Page column 33
[5] Journal of Organic Chemistry, 1963, vol. 28, p. 1816 - 1821 |
|
|