101711-78-8
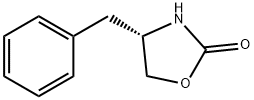
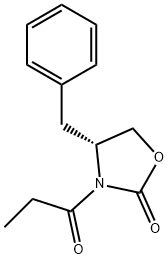
 101711-78-8 结构式
101711-78-8 结构式
基本信息
(S)-(+)-4-苄基-3-丙酰基-2-恶唑烷酮
N-3-PROPIONYL-(4S)-BENZYL-2-OXAZOLIDINONE
N-PROPIONYL-(4S)-BENZYL-2-OXAZOLIDINONE
(S)-(+)-4-BENZYL-3-PROPIONYL-2-OXAZOLIDINONE
(S)-4-BENZYL-3-PROPIONYL-2-OXAZOLIDINONE
(S)-(+)-4-Benzyl-3-propiomyl-2-oxazolidinone
(S)-(+)-4-BENZYL-3-PROPIONYL-2-OXAZOLIDI N-ONE, 99% (99% EE/HPLC)
(4s)-(+)-benzyl-3-propionyl-2-oxazolidinone
(S)-(+)-4-BENZYL-3-PROPIONYL-2-OXALIDINONE
物理化学性质
安全数据
制备方法
90719-32-7

79-03-8
131685-53-5
在氮气氛围下,将(S)-4-苄基-2-唑烷酮(800 mg,4.5 mmol)溶于无水四氢呋喃(30 mL)的干燥反应瓶中,并在干冰/丙酮浴中冷却至-78℃。在3分钟内缓慢滴加1.6 M正丁基锂的己烷溶液(5.0 mmol,3.1 mL)。搅拌30分钟后,在3分钟内滴加丙酰氯(5.0 mmol,0.43 mL)。反应液在14小时内缓慢升温至室温,随后通过加入饱和氯化铵溶液(10 mL)和水(30 mL)淬灭反应。水层用乙酸乙酯(3×40 mL)萃取,合并有机相并用无水硫酸镁干燥,减压浓缩后得到(R)-(-)-苄基-3-丙酰基-2-恶唑烷酮(油状物,1.0 g,收率96%),HPLC纯度>95%。1H NMR(500 MHz,CDCl3):δ7.33(dd,J1=7.4 Hz,J2=7.6 Hz,2H),7.29(d,J=6.9 Hz,1H),7.21(d,J=7.4 Hz,2H),4.67(m,1H),4.18(m,2H),3.31(dd,J1=13.3 Hz,J2=3.2 Hz,1H),2.96(m,2H),2.77(dd,J1=13.3 Hz,J2=3.7 Hz,1H),1.21(t,J=7.4 Hz,3H)。
参考文献:
[1] Chemistry - An Asian Journal, 2011, vol. 6, # 7, p. 1791 - 1799
[2] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 1, p. 144 - 148
[3] Journal of Organic Chemistry, 2008, vol. 73, # 8, p. 3292 - 3294
[4] Angewandte Chemie - International Edition, 2016, vol. 55, # 13, p. 4252 - 4255
[5] Angew. Chem., 2016, vol. 128, # 13, p. 4324 - 4327,4
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | B2166 | (S)-(+)-4-苄基-3-丙酰-2-恶唑烷酮 (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone | 101711-78-8 | 1G | 165元 |
| 2025/12/22 | B2166 | (S)-(+)-4-苄基-3-丙酰-2-恶唑烷酮 (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone | 101711-78-8 | 5G | 565元 |
| 2025/05/22 | XW0210171178804 | (S)-4-苄基-3-丙酰基-2-噁唑烷酮 | 101711-78-8 | 100G | 675元 |

