113028-17-4
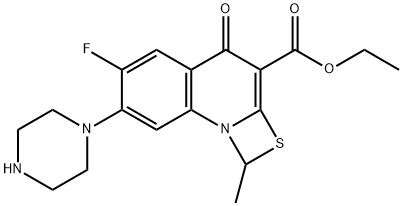 113028-17-4 结构式
113028-17-4 结构式
基本信息
普卢利沙星中间体二
普卢利沙星中间体PL-10
普卢利沙星PL-10
ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4h-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate
PRULIFLOXACIN INTERMEDIATE 2
ETHYL 6-FLUORO-1-METHYL-4-OXO-7-(1-PIPRAZINYL)-4H-(1,3)THIAZETO(3,2-A)QUINOLINE-
Ethyl 6-Fluoro-1-Methyl-4-Oxo-7-91-Piperazinyl)-4h-(1,3-)-Thiazeto-(3,2-A)Quinoline-3-Carboxylate
6-FLUORO-1-METHYL-4-OXO-7-(1-PIPERAZINYL)-4H-(1,3)THIAZETO(3,2-A)QUINOLINE-3-CARBOYLIC ACID ETHYL ESTER
6-FLUORO-1-METHYL-4-OXO-7-(1-PIPRAZINYL)-1H,4H-(1,3)THIAZETO(3,2-A)QUINOLINE-3-CARBOXYLIC ACID, ETHYL ESTER
6-Fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-1H,4H-[1,3]-thiazeto[3,2-a]-3-quinolinecarboxylic acid ethyl ester
ETHYL 6-FLUORO-1-METHYL-4-OXO-7-(1-PIPERAZINYL)-4H-(1,3)-THIAZETO(3,2-A) QUINOLINE-3-CARBOXYLATE
Prulifloxacin Intermediate PL-10
物理化学性质
制备方法

110-85-0
![6,7-二氟-1-甲基-4-氧代-4H-[1,3]噻嗪[3,2-a]并喹啉-3-羧酸乙酯](/CAS/GIF/113046-72-3.gif)
113046-72-3
![6-氟-7-哌嗪-1-甲基-4-氧代-[1,3]硫氮杂环[3,2-a]喹啉-3-羧酸乙酯](/CAS/GIF/113028-17-4.gif)
113028-17-4
以6,7-二氟-1-甲基-4-氧代-1,4-二氢-[1,3]硫氮杂丁环并[3,2-a]喹啉-3-羧酸乙酯和哌嗪为原料,合成6-氟-7-哌嗪-1-甲基-4-氧代-[1,3]硫氮杂环[3,2-a]喹啉-3-羧酸乙酯的一般步骤如下:将50g 6,7-二氟-1-甲基-4-氧代-4H-[1,3]噻嗪[3,2-a]喹啉-3-羧酸乙酯溶于5体积的DMSO中,加热至60℃。随后加入40g哌嗪,保持60℃搅拌反应4小时。反应完成后,将混合物冷却至室温,加入5体积的乙腈,继续在室温下搅拌4小时。过滤收集沉淀,干燥后得到52.8g 6-氟-7-哌嗪-1-甲基-4-氧代-[1,3]噻唑环[3,2-a]喹啉-3-羧酸乙酯,收率为87%。进一步纯化后,6-氟-7-哌嗪-1-甲基-4-氧代-[1,3]噻唑环[3,2-a]喹啉-3-羧酸乙酯的收率可达99%。
参考文献:
[1] Patent: CN107383069, 2017, A. Location in patent: Paragraph 0061; 0062
[2] Patent: CN107501298, 2017, A. Location in patent: Paragraph 0055; 0056
[3] Journal of Medicinal Chemistry, 1992, vol. 35, # 25, p. 4727 - 4738
[4] Patent: US4843070, 1989, A
[5] Patent: WO2009/93268, 2009, A1. Location in patent: Page/Page column 15