125995-13-3
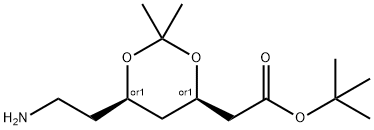
 125995-13-3 结构式
125995-13-3 结构式
基本信息
6-氨乙基-2,2-二甲基-1,3-二氧戊环-乙酸叔丁酯
ATROVASTATIN INTERMEDIATES
6-氨乙基-2,2-二甲基-1,3-二氧戊环-4-乙酸叔丁酯
侧链ATS-9
(4R,6R)-叔-丁基-6-(2-氨基乙基)-2,2-二甲基1,3-二噁烷-4-乙酸
(4R,6R)-TERT-BUTYL-6-(2-AMINOETHYL)-2,2-DIMETHYL-1,3-DIOXANE-4-ACETATE
(4R,CIS)-1,1-DIMETHYLETHYL-6-AMINOETHYL-2,2-DIMETHYL-1,3-DIOXANE-4-ACETATE
(4R-Cis)-1,1-Dimethylethyl-6-aminoethyl-2,2-dimethyl-1,3-dioxoane-4-acetate
ATS-9
TERT-BUTYL[(4R,6R)-6-(2-AMINOETHYL)-2,2-DIMETHYL-1, 3-DIOXAN-4-YL]ACETATE
(4R-CIS)-1,1-DIMETHYLETHYL-6-AMINOETHYL-2,2-DIMETHYL-1,3-DIOXANE-4-ACETATE (ATS-9)
Atorvastatin Intermediate Ⅰ
Atorvastatin Intermediate 1
(4R-Cia)-1,1-dimethyl ethyl-6-cyano methyl-2,2-dimethyl-1,3-dioxane-4-acetate
(4R,Cis)-1,1-Dimethylethyl-6-(2-Aminoethyl)-2,2-Dimethyl-1,3-Dioxane-4-Acetate
(4R-cis)-1,1-dimethylethyl-6-aminoethyl- 2,2-dimethyl-1,3-dloxane-4-acetate
(4R,6R)-6-(2-aminoethyl)-2,2-dimethyl-1,3-Dioxane-4-acetic acid, 1,1-dimethylethyl ester
(4R, 6R)-6-(2aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-Butyl Easter
(4R,6R)-Tert-Butyl-6-(2-Aminoe
ATS 9 (4R,CIS)-1,1-DIMETHYLETHYL-6-AMINOETHYL-2,2-DIMETHYL-1,3-DIOXANE-4-ACETATE
TERT-BUTYL 2-((4R,6S)-6-(AMINOMETHYL)-2,2-DIMETHYL-1,3-DIOXAN-4-YL)ACETATE
(4R-Cis)-1,1-dimethylethyl-6-aminomethyl-2,2-dimethyl-1,3-dio-cxane-acetate
ATS-9 (4R, cis)C1,1-dimethylethyl-6-aminoethyl-2,2-dimethyl-1,3-dioxane C4-Acetate
(4R, 6R)-6-(2aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-Butyl Ester
物理化学性质
制备方法

125971-94-0
125971-86-0
以(4R-cis)-6-氰甲基-2,2-二甲基-1,3-二氧六环-4-乙酸叔丁酯为原料合成目标化合物(CAS:125971-86-0)的一般步骤:向5加仑不锈钢反应器中加入250g雷尼镍(Ra-Ni)、(4R,6R)-6-氰基甲基-2,2-二甲基-[1,3]二恶烷-4-基-乙酸叔丁酯(1.0kg,3.71mol)、甲苯(6L)、甲醇(675mL)及6.5M氨/甲醇溶液(800mL)。密封反应器,用氮气(N2)进行压力测试至3.5巴,并用3.5巴的N2吹扫三次。随后,用氢气(H2)将反应器吹扫三次至3.5巴,此过程中不开启搅拌。在H2加压至3.5巴后,启动搅拌,反应2-6小时,观察到轻微放热,温度升至30-40℃。持续搅拌直至H2吸收停止,然后在30至40℃下继续搅拌30分钟。将反应混合物冷却至20-25℃,关闭H2源和搅拌器,排出反应器中的H2。重新启动搅拌器,用N2吹扫反应器三次至3.5巴。在氮气保护下过滤回收的镍催化剂,并用甲苯(250mL)洗涤反应器和催化剂床。合并滤液,在不超过55℃的条件下减压浓缩至约500mL。[注意:浓缩完成后用氮气破坏真空]。向浓缩液中加入饱和氯化钠溶液,在氮气保护下搅拌10分钟。停止搅拌,静置分层。弃去下层水相,有机相经浓缩得到黄色油状目标产物(1.054kg,收率104%,含7%残留甲苯);1H-NMR(400MHz,CDCl3):δ4.23-4.19(m,1H),3.99-3.95(m,1H),2.74(t,J=7.1Hz,2H),2.40-2.36(m,1H),2.27-2.22(m,1H),1.58-1.41(m,2H),1.40(s,9H),1.31(s,6H),0.89(s,9H);低分辨率质谱(APCI)m/z 273 [M+H]+。
参考文献:
[1] Patent: US2005/239857, 2005, A1. Location in patent: Page/Page column 83
[2] Patent: US8563753, 2013, B2. Location in patent: Page/Page column 18; 19
[3] Patent: WO2007/49121, 2007, A1. Location in patent: Page/Page column 17-18
[4] Patent: CN104230880, 2016, B. Location in patent: Paragraph 0066-0067
[5] Tetrahedron Letters, 1992, vol. 33, # 17, p. 2283 - 2284
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | B3702 | 2-[(4R,6R)-6-(2-氨乙基)-2,2-二甲基-1,3-二恶烷-4-基]乙酸叔丁酯 tert-Butyl 2-[(4R,6R)-6-(2-Aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetate | 125995-13-3 | 1g | 70元 |
| 2025/12/22 | B3702 | 2-[(4R,6R)-6-(2-氨乙基)-2,2-二甲基-1,3-二恶烷-4-基]乙酸叔丁酯 tert-Butyl 2-[(4R,6R)-6-(2-Aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetate | 125995-13-3 | 5g | 210元 |
| 2024/08/19 | XW1259951334 | (4R,6R)-2,2-二甲基-6-(2-氨乙基)-1,3-二氧六环-4-乙酸叔丁酯 (4r,6r)-tert-butyl 6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetate;(4r,6r)-tert-butyl-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetate;tert-butyl 2-[(4r,6r)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetate;(4r-cis)-1,1-dimethylethyl-6-aminoethyl-2,2-dimethyl-1,3-dioxoane-4-acetate;2-[(4r,6r)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester | 125995-13-3 | 100G | 405元 |
