161796-10-7
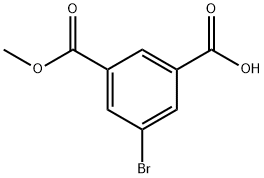 161796-10-7 结构式
161796-10-7 结构式
基本信息
3-溴-5-甲氧羰基苯甲酸
3-溴-5-(甲氧基羰基)苯甲酸
3-Bromo-5-(methoxycarbonyl)
Methyl 5-broMoisophthalic acid
monomethyl 5-bromoisophthalate
5-TERT-BUTYL METHYLISOPHTHALATE
3-TERT-BUTYL METHYLISOPHTHALATE
5-TERT-BUTYL DIMETHYLISOPHTHALATE
3-BROMO-5-(METHOXYCARBONYL)BENZOIC ACID
METHYL 5-TERT-BUTYL-ISOPHTHALATE DIESTER
5-BROMOISOPHTHALIC ACID MONOMETHYL ESTER
物理化学性质
制备方法

28179-47-7

161796-10-7
步骤G(2):3-溴-5-(甲氧基羰基)苯甲酸的合成。 在0℃下,向含有溴化铜(II)(5.5g,24.6mmol)、亚硝酸叔丁酯(3.17g,30.75mmol)和乙腈(300mL)的混合溶液中,缓慢加入3-氨基-5-(甲氧基羰基)苯甲酸(4.0g,20.5mmol)的乙腈(300mL)溶液,加料时间为30分钟。随后,将反应混合物升温至室温并继续搅拌3小时。反应完成后,加入水并减压蒸馏除去乙腈。向残余物中加入乙酸乙酯(600mL),依次用3N HCl、水洗涤有机层,用无水Na2SO4干燥,减压浓缩,得到目标产物3-溴-5-羧基苯甲酸甲酯,收率97%。产物表征数据如下:1H NMR (CDCl3, 500 MHz) δ 3.96 (3H, s), 8.41 (1H, s), 8.67 (1H, s); MS (ESI) m/z 259.02 (M-H)-。
参考文献:
[1] Patent: WO2006/99352, 2006, A1. Location in patent: Page/Page column 41
[2] Journal of the American Chemical Society, 2003, vol. 125, # 34, p. 10241 - 10249
[3] Patent: WO2009/15166, 2009, A1. Location in patent: Page/Page column 87-88
[4] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 10, p. 2654 - 2660
[5] Patent: US2006/223759, 2006, A1. Location in patent: Page/Page column 193
