165800-06-6
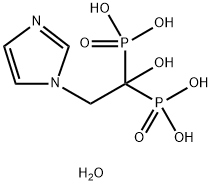 165800-06-6 结构式
165800-06-6 结构式
基本信息
唑来磷酸一水化合物
(1-hydroxy-2-imidazol-1-yl-1-phosphono-ethyl)phosphonic acid hydrate
(1-hydroxy-2-imidazol-1-ylethylidene)diphosphonic acid monohydrate
PHOSPHONIC ACID, [1-HYDROXY-2-(1H-IMIDAZOL-1-YL)ETHYLIDENE]BIS-, MONOHYDRATE
zoledronic acid hydrate
ZOLEDRONIC ACID MONOHYDRATE
ZoledronicacidforInjection
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法

118072-93-8

165800-06-6
以(1-羟基-2-(1H-咪唑-1-基)乙烷-1,1-二基)二膦酸为原料合成1-羟基-2-(1-咪唑基)乙烷-1,1-二磷酸水合物的一般步骤如下: 实施例3:唑来膦酸一水合物的制备 将根据实施例1获得的原始潮湿唑来膦酸(相当于90.3g粗产物)悬浮于3050ml水中。在搅拌条件下,将悬浮液加热至回流状态。随后,加入EPO水使总体积达到3750ml,直至完全溶解。停止加热,继续搅拌,使溶液缓慢冷却至室温。当内部温度降至约70-80℃时,进一步冷却至2-5℃,并在此温度下维持1小时。过滤沉淀,并用冰水洗涤。将所得沉淀置于气流烘箱中干燥。最终,在5°C至60°C条件下获得169.3g(产率89%)无色晶体。通过热重分析(TGA)测得结晶水损失为6.8%,证实为一水合物。该物质的X射线粉末衍射(XRPD)图谱在2θ值为12.1°、12.8°、15.7°、18.9°±0.2°处显示特征峰,与已知晶型I(US 2005/0054616)的衍射数据一致。图1A展示了其XRPD图谱,图IV则展示了晶胞中原子的排列结构。
参考文献:
[1] Patent: WO2007/16982, 2007, A1. Location in patent: Page/Page column 7-8
[2] Patent: EP1925621, 2008, A1. Location in patent: Page/Page column 14
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-13777A | 唑来磷酸一水化合物 Zoledronic acid monohydrate | 165800-06-6 | 50mg | 800元 |
| 2025/12/22 | HY-13777A | 唑来磷酸一水化合物 Zoledronic acid monohydrate | 165800-06-6 | 100mg | 1200元 |
| 2025/12/22 | Z0031 | 唑来膦酸 一水合物 Zoledronic Acid Monohydrate | 165800-06-6 | 1g | 340元 |
常见问题列表
| Target | Value |
|
Ras
() | |
|
Rho
() |
Zoledronic Acid monohydrate (0.1-1 µM; 48 hours) increases receptor activator of nuclear factor kB ligand (RANKL) and sclerostin mRNA expressions in osteocyte-like MLO-Y4 cells.
Zoledronic Acid monohydrate increases the expression of osteoclastogenesis supporting factor from MLO-Y4 cells.
Zoledronic Acid monohydrate enhances the RANKL expression via IL-6/ JAK2/STAT3 pathway in MLO-Y4 cells.
Zoledronic Acid monohydrate inhibits osteoclast differentiation and function through the regulation of NF-κB and JNK signalling pathways.
Zoledronic Acid monohydrate (10-100 µM; 1-7 days) markedly reduces the viability of MC3T3-E1 cells.
Zoledronic Acid monohydrate (10-100 µM; 1-7 days) induces apoptosis in MC3T3-E1 cells.
Zoledronic Acid monohydrate (10-100 µM; 4 days) inhibits cell viability due to the induction of apoptosis.
Zoledronic Acid monohydrate exerts inhibitory effects on the differentiation and maturation of MC3T3-E1 cells at concentrations <1 µM.
Cell Viability Assay
| Cell Line: | MC3T3-E1 cells |
| Concentration: | 0.02 µM , 0.1 µM, 1 µM, 10 µM, 100 µM |
| Incubation Time: | 1 day, 3 days, 5 days, 7 days |
| Result: | Reduced cells viability at 10 µM and 100 µM. |
Apoptosis Analysis
| Cell Line: | MC3T3-E1 cells |
| Concentration: | 0.02 µM , 0.1 µM, 1 µM, 10 µM, 100 µM |
| Incubation Time: | 1 days, 4 days, 7 days |
| Result: | Increased the number of early apoptotic cells and late apoptotic or necrotic cells at dose-dependent and time-dependent (high concentrations). |
Western Blot Analysis
| Cell Line: | MC3T3-E1 cells |
| Concentration: | 0.02 µM , 0.1 µM, 1 µM, 10 µM, 100 µM |
| Incubation Time: | 4 days |
| Result: | Down-regulated the protein level of inactive caspase-3 and up-regulated the protein level of active caspase-3 at the concentrations of 10 and 100 µM. |
Zoledronic Acid monohydrate (0.05 mg/kg; i.p.; weekly; for 3 weeks) increases bone mineral density and content.
Zoledronic Acid monohydrate (0.5-1 mg/kg; i.p.; weekly; for 3 weeks) inhibits both osteoclast and osteoblasts function and bone remodeling in vivo interfering with bone mechanical properties.
| Animal Model: | Five-week-old C57BL6 mice |
| Dosage: | 0.05 mg/kg, 0.5 mg/kg, 1 mg/kg |
| Administration: | Intraperitoneal injection, weekly, for 3 weeks |
| Result: | Inhibited both osteoclast and osteoblasts function and bone remodeling at 0.5 mg/kg and 1 mg/kg. |
