175213-46-4
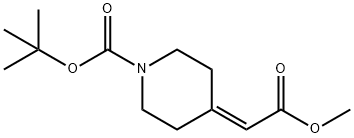
 175213-46-4 结构式
175213-46-4 结构式
基本信息
1-BOC-4-哌啶乙酸甲酯
N-BOC-哌啶-4-乙酸甲酯
11-BOC-4-哌啶乙酸甲脂
1-N-BOC-4-哌啶乙酸甲酯
1-N-BOC-4-哌啶乙酸甲酯
N-叔丁氧羰基-4-哌啶乙酸甲酯
METHYL 1-BOC-4-PIPERIDINEACETATE
Methyl N-Boc-4-piperidineacetate
Methyl N-BOC-piperidine-4-acetate
Methyl 1-N-Boc-4-piperidineacetate
1-Boc-4-Piperidine acetate methyl ester
N-BOC-4-PIPERIDINEACETIC ACID METHYL ESTER
1-Boc-4-Piperidine acetic acid methyl ester
Methyl (piperidin-4-yl)acetate, N-BOC protected
2-{1-[(tert-butoxy)carbonyl]piperidin-4-yl}propanoate
物理化学性质
制备方法
169206-65-9

175213-46-4
以4-(2-甲氧基-2-氧代亚乙基)哌啶-1-甲酸叔丁酯为原料合成N-Boc-4-哌啶乙酸甲酯的一般步骤:将1-叔丁氧基羰基-4-[(甲氧基羰基)亚甲基]哌啶(875 mg)溶于乙醇(10 mL)中,向该溶液中加入10%钯碳催化剂(约50%水含量,730 mg)。将反应混合物在室温及常压下进行催化氢化反应3天。反应完成后,通过过滤去除催化剂,随后在减压条件下蒸馏除去溶剂,得到目标产物N-Boc-4-哌啶乙酸甲酯(871 mg,收率99%)。产物经1H-NMR(CDCl3)表征:δ 1.16(2H,m),1.45(9H,s),1.65(2H,m),1.93(1H,m),2.25(2H,d,J = 6.8 Hz),2.72(2H,br),3.68(3H,s),4.08(2H,br)。质谱(FAB)分析显示m/z:258(M + H)+。
参考文献:
[1] Patent: EP1104754, 2001, A1
[2] Patent: EP1577302, 2005, A1. Location in patent: Page/Page column 121-122
[3] Patent: US2006/94707, 2006, A1. Location in patent: Page/Page column 43
[4] Patent: US2007/259850, 2007, A1. Location in patent: Page/Page column 79
[5] Patent: WO2011/123232, 2011, A1. Location in patent: Page/Page column 9
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0217073753805 | 4-(2-甲氧基-2-氧代乙基)哌啶-1-羧酸苄酯 Benzyl 4-(2-methoxy-2-oxoethyl)piperidine-1-carboxylate | 175213-46-4 | 25g | 1412元 |
| 2025/12/22 | XW0217073753804 | 4-(2-甲氧基-2-氧代乙基)哌啶-1-羧酸苄酯 Benzyl 4-(2-methoxy-2-oxoethyl)piperidine-1-carboxylate | 175213-46-4 | 10g | 703元 |
| 2025/12/22 | XW0217073753803 | 4-(2-甲氧基-2-氧代乙基)哌啶-1-羧酸苄酯 Benzyl 4-(2-methoxy-2-oxoethyl)piperidine-1-carboxylate | 175213-46-4 | 5g | 378元 |
