189028-93-1
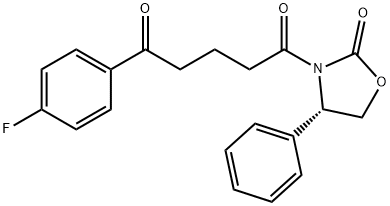 189028-93-1 结构式
189028-93-1 结构式
基本信息
依替米贝中间体 3
3-[5-[(4-FLUOROPHENYL)-1-5-DIOXOPENTA]-YL-4-(S)-PHENYL OXNZOLIDINE-2-ONE
3-[5-(4-FLUOROPHENYL)-1,5-DIOXOPENTYL]-4-PHENYL-(4S)-2-OXAZOLIDINONE
(4s)-3-[5-(4-fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone
3-[5-(4-Fluorophenyl)-1,5-Dixopenyl]-4-Phenyl-(4s)-2-Oxazolidinone
(4S)-3-[5-(4-fluorophenyl)1,5-dioxophentyl]-4-phenyl-2-oxazolidinone
API`sIntermediate
(4S)-3-[5-(4-Fluorophenyl)-1,5-dixopenyl]-4-phenyl-2-oxazolidinone
2-OXAZOLIDINONE,3-[5-(4-FLUOROPHENYL)-1,5-DIOXOPENTYL]-4-PHENYL
物理化学性质
制备方法

99395-88-7
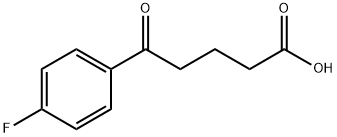
149437-76-3
![(4S)-3-[5-(4-氟苯基)-1,5-二氧代戊基]-4-苯基-2-恶唑烷酮](/CAS/GIF/189028-93-1.gif)
189028-93-1
实施例1:依泽替米贝的制备; (1-1)(4S)-3-[5-(4-氟苯基)-1,5-二氧代戊基]-4-苯基-2-恶唑烷酮(式7)的制备;将200g 5-(4-氟苯基)-5-氧代戊酸(式8),160g (S)-4-苯基恶唑烷-2-酮(式9)和11.6g 4-二甲基氨基吡啶溶解在600mL二氯甲烷中制备反应混合物。将通过将157g N,N'-二环己基碳酰亚胺溶解在200mL二氯甲烷中制备的溶液加入到反应混合物中并搅拌2小时。反应完成后,过滤所得反应混合物以除去副产物。将滤液依次用1L 6N HCl、1L水和1L饱和氯化钠溶液洗涤,用无水硫酸镁干燥,过滤,减压蒸馏除去溶剂。将残余物通过加热溶解在2L甲醇中并冷却以诱导结晶。加入2L水并搅拌30分钟。通过过滤分离固体,得到289g (4S)-3-[5-(4-氟苯基)-1,5-二氧代戊基]-4-苯基-2-恶唑烷酮,为白色固体(收率:86%)。1H NMR(300MHz,CDCl3):δ7.92(2H,m),7.35-7.13(5H,m),7.04(2H,m),5.43(1H,q),4.75(1H,t),4.22(1H,q),3.05-2.93(4H,m),2.03(2H,m)。
参考文献:
[1] Patent: WO2010/71358, 2010, A2. Location in patent: Page/Page column 14-15
[2] Patent: WO2006/122216, 2006, A2. Location in patent: Page/Page column 41-42
[3] Synthetic Communications, 2016, vol. 46, # 20, p. 1687 - 1693
[4] Patent: CN106397292, 2017, A. Location in patent: Paragraph 0053; 0086; 0087
[5] Patent: CN104513187, 2017, B. Location in patent: Paragraph 0063; 0064; 0065; 0066
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | P2111 | (S)-4-苯基-3-[5-(4-氟苯基)-5-氧代戊酰基]-2-恶唑烷酮 (S)-4-Phenyl-3-[5-(4-fluorophenyl)-5-oxopentanoyl]-2-oxazolidinone | 189028-93-1 | 5g | 120元 |
| 2025/12/22 | P2111 | (S)-4-苯基-3-[5-(4-氟苯基)-5-氧代戊酰基]-2-恶唑烷酮 (S)-4-Phenyl-3-[5-(4-fluorophenyl)-5-oxopentanoyl]-2-oxazolidinone | 189028-93-1 | 25g | 400元 |
| 2025/05/22 | XW1890289313 | (4S)-3-[5-(4-氟苯基)-1,5-二氧代戊基]-4-苯基-2-恶唑烷酮 | 189028-93-1 | 100G | 780元 |