24764-66-7
 24764-66-7 结构式
24764-66-7 结构式
基本信息
4-甲氧基-1-萘乙酮
1-乙酰基-4-甲氧基萘
1-(4-甲氧基-1-萘基)乙酮
1-(4-甲氧基萘-1-基)乙酮
4-甲氧基-1-萘乙酮(4-甲氧基萘乙酮)
4-甲氧基萘乙酮(CAS号:24764-66-7)
1-(4-METHOXY-1-PHTHYL)ETHANONE
1-(4-Methoxy-1-naphthyl)ethanone
4-Methoxy-1-Naphthyl Ethyl Ketone
1-(4-methoxynaphthalen-1-yl)ethanone
1-(4-Methoxy-1-naphthalenyl)-ethanone
Ethanone, 1-(4-methoxy-1-naphthalenyl)-
制备方法
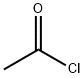
75-36-5
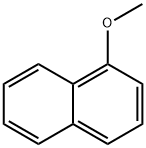
2216-69-5

24764-66-7
在氮气保护下,将无水二氯甲烷(12 mL)中的三氯化铝(800 mg,6 mmol)冷却至0℃,缓慢加入乙酰氯(236 mg,3 mmol)。将反应混合物于室温下搅拌10分钟后,加入1-甲氧基萘(474 mg,3 mmol),继续在室温下搅拌过夜。反应完成后,用水淬灭反应,分离有机相,用无水硫酸钠干燥,减压浓缩。残余物通过硅胶柱色谱纯化(洗脱剂:乙酸乙酯/石油醚=1:15,v/v),得到4-甲氧基萘乙酮(338 mg,收率56%)为白色固体。1H NMR (300 MHz, CDCl3) δ: 9.01-9.96 (m, 1H), 8.32-8.35 (m, 1H), 8.03 (d, 1H), 7.63-7.65 (m, 1H), 7.53-7.58 (m, 1H), 6.79 (d, 1H), 4.07 (s, 3H), 2.72 (s, 3H)。LC-MS: m/z 201 [M+1]+。
参考文献:
[1] Canadian Journal of Chemistry, 1981, vol. 59, p. 2629 - 2641
[2] Patent: US2012/220629, 2012, A1. Location in patent: Page/Page column 46
[3] Patent: WO2012/113103, 2012, A1. Location in patent: Page/Page column 62-63
[4] Recueil des Travaux Chimiques des Pays-Bas, 1945, vol. 64, p. 214,216
[5] Zhurnal Obshchei Khimii, 1938, vol. 8, p. 402,407
