39945-51-2
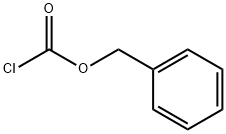
 39945-51-2 结构式
39945-51-2 结构式
基本信息
1-CBZ-3-HYDROXYMETHYL-PIPERIDINE
3-HYDROXYMETHYL-PIPERIDINE-1-CARBOXYLIC ACID BENZYL ESTER
BENZYL 3-(HYDROXYMETHYL)PIPERIDINE-1-CARBOXYLATE
BENZYL 3-(HYDROXYMETHYL)TETRAHYDRO-1(2H)-PYRIDINECARBOXYLATE
BUTTPARK 75\50-54
N-Cbz-3-hydroxymethylpiperidine
物理化学性质
制备方法

4606-65-9
501-53-1

39945-51-2
向3-羟甲基哌啶(0.61 g, 5 mmol)的二氯甲烷(10 mL)冷冻溶液中加入三乙胺(0.51 g, 5 mmol),随后缓慢滴加氯甲酸苄酯(0.88 g, 5 mmol)。将反应混合物逐渐升温至室温并持续搅拌过夜。反应完成后,加入二氯甲烷(50 mL)稀释反应混合物,依次用5%盐酸溶液(2 × 20 mL)、饱和碳酸氢钠溶液(20 mL)和饱和氯化钠溶液(20 mL)洗涤有机层。有机层经无水硫酸钠干燥后,过滤除去干燥剂,减压浓缩去除溶剂。粗产物通过快速柱色谱法(硅胶,乙酸乙酯/己烷为洗脱剂)纯化,得到目标化合物3-甲醇-N-苄氧羰基哌啶(1.21 g, 收率96%),为无色油状物。1H NMR (CDCl3) δ: 1.20-1.83 (m, 5H), 2.78-3.20 (m, 2H), 3.50 (s, 2H), 3.69-4.05 (m, 2H), 5.14 (s, 2H), 7.25-7.40 (m, 5H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 20, p. 5975 - 5983
[2] Journal of Medicinal Chemistry, 2006, vol. 49, # 9, p. 2673 - 2676
[3] Patent: WO2005/806, 2005, A2. Location in patent: Page 85
[4] Patent: US2010/48606, 2010, A1. Location in patent: Page/Page column 32
[5] Patent: EP1780210, 2007, A1. Location in patent: Page/Page column 51
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0240506152101 | (3S)-3-(羟甲基)哌啶-1-羧酸苄酯 benzyl (3S)-3-(hydroxymethyl)piperidine-1-carboxylate | 39945-51-2 | 100mg | 95元 |
| 2025/12/22 | XW023994551204 | N-Cbz-3-哌啶甲醇 Benzyl 3-(hydroxymethyl)piperidine-1-carboxylate | 39945-51-2 | 25g | 443元 |
| 2025/12/22 | XW023994551203 | N-Cbz-3-哌啶甲醇 Benzyl 3-(hydroxymethyl)piperidine-1-carboxylate | 39945-51-2 | 10g | 204元 |
