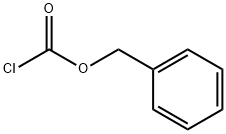
122860-33-7
 122860-33-7 结构式
122860-33-7 结构式
基本信息
N-CBZ-4-羟甲基哌啶
N-苄氧羰基-4-哌啶甲醇
N-CBZ-4-哌啶甲醇
1-N-CBZ-HYDROXYMETHYL-PIPERIDINE
4-HYDROXYMETHYL-PIPERIDINE-1-CARBOXYLIC ACID BENZYL ESTER
BENZYL 4-(HYDROXYMETHYL)PIPERIDINE-1-CARBOXYLATE
BENZYL 4-(HYDROXYMETHYL)TETRAHYDRO-1(2H)-PYRIDINECARBOXYLATE
BUTTPARK 75\50-51
N-(BENZYLOXYCARBONYL)-4-(HYDROXYMETHYL)PIPERIDINE
N-CARBOBENZOXY-4-PIPECOLINOL
N-CARBOBENZOXY-4-PIPERIDINEMETHANOL
N-CARBOBENZOXY-HEXAHYDROISONICOTINOL
Z-ISONICOT(H6)-OL
Z-ISONIPECOTINOL
Z-PIC(4)-OL
1-N-Cbz-4-Hydroxymethyl-piperidine
N-Cbz-4-(hydroxymethyl)piperidine
N-(Benzyloxycarbonyl)-4-(Hydroxymethyl)piperidine, 97 %
物理化学性质
制备方法

6457-49-4
501-53-1

122860-33-7
以4-羟甲基哌啶和氯甲酸苄酯为原料合成N-CBZ-4-哌啶甲醇(1-CBZ-4-羟甲基哌啶)的一般步骤:在无水二氯甲烷(DCM, 100 mL)中溶解4-羟甲基哌啶(2.0 g, 17.4 mmol),将溶液冷却至0℃并搅拌。依次加入三乙胺(4.8 mL, 34.8 mmol)和氯甲酸苄酯(3.7 mL, 34.8 mmol),然后将反应混合物缓慢升温至室温并继续搅拌2小时。反应完成后,将混合物转移至分液漏斗中,加入DCM(50 mL)和水(30 mL)进行分层。分离有机相,水相用DCM(2×50 mL)萃取。合并所有有机相,用盐水(30 mL)洗涤一次,经无水硫酸钠干燥后,减压浓缩得到粗产物。粗产物通过柱色谱法纯化,使用己烷/EtOAc(70%至100%)梯度洗脱,得到3.85 g(89%产率)的N-苄氧基羰基-4-(羟甲基)哌啶(E-12),为透明油状物。其1H NMR(CDCl3)数据如下:δ 1.17(m, 2H), 1.72(m, 3H), 2.15(br s, 1H), 2.78(t, 2H, J = 12 Hz), 3.47(d, 2H, J = 6.04 Hz), 4.20(d, 2H, J = 11.68 Hz), 5.12(s, 2H), 7.33(m, 5H)。
参考文献:
[1] Patent: WO2005/80394, 2005, A1. Location in patent: Page/Page column 131
[2] European Journal of Organic Chemistry, 2008, # 25, p. 4277 - 4295
[3] Patent: WO2010/46780, 2010, A2. Location in patent: Page/Page column 63-64
[4] Journal of the American Chemical Society, 2017, vol. 139, # 24, p. 8110 - 8113
[5] Patent: US2003/55244, 2003, A1
