41789-95-1
 41789-95-1 结构式
41789-95-1 结构式
基本信息
3-甲氧基-N-甲基苄胺
N-甲基-3-甲氧基苄胺
3-甲氧基-N-甲基苯甲胺
3-甲氧基-N-甲基苯甲基胺
(3-甲氧基苄基)-甲基-胺
(3-甲氧基苄基)甲胺盐酸盐
N-甲基-3-甲氧基苄胺 10G
1-(3-甲氧苯基)-N-甲基甲胺
1-(3-甲氧苯基)-N-甲基-甲胺
Diclofensine Impurity 1
(3-methoxybenzyl)methylamine
N-Methyl-3-MethoxybenzylaMine
3-Methoxy-N-methylbenzylamine
3-METHOXY-N-METHYLBENZYLAMINE 97
N-(3-METHOXYBENZYL)-N-METHYLAMINE
(3-Methoxyphenyl)-N-MethylMethanaMine
3-Methoxy-1-(MethylaMinoMethyl)benzene
[(3-Methoxyphenyl)Methyl](Methyl)aMine
物理化学性质
安全数据
Eye Dam. 1
Skin Sens. 1
制备方法

591-31-1
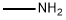
74-89-5

41789-95-1
向3-甲氧基苯甲醛(180 g,1.32 mol)的甲醇(1 L)溶液中缓慢加入40%甲胺水溶液(113 mL,1.31 mol),反应混合物于0℃下搅拌1小时。随后,在相同温度下分批加入硼氢化钠(75 g,1.98 mol),继续搅拌1小时。反应完成后,将溶液减压浓缩至较小体积,用水(200 mL)稀释,并用二氯甲烷(3×500 mL)萃取。合并有机相,经无水硫酸钠干燥,过滤后减压浓缩,得到粗产物N-甲基-3-甲氧基苄胺(220 g,定量),为透明油状物,直接用于后续反应无需进一步纯化。1H NMR (CDCl3, 500 MHz) δ: 7.23 (t, J = 8.0 Hz, 1H), 6.92-6.88 (m, 2H), 6.81-6.78 (m, 1H), 3.80 (s, 3H), 3.73 (s, 2H), 2.45 (s, 3H), 2.07 (br s, 1H)。
参考文献:
[1] Patent: WO2010/132437, 2010, A1. Location in patent: Page/Page column 74; 101-102
[2] Patent: WO2009/149258, 2009, A2. Location in patent: Page/Page column 19; 23
[3] Patent: US2007/21408, 2007, A1. Location in patent: Page/Page column 57; 59; 60; 62; 63; 64-65; 66; 70; 71; 73; 74; 88
[4] Patent: US2014/275101, 2014, A1. Location in patent: Paragraph 0072; 0074; 0075
[5] Synthesis, 1990, # 3, p. 253 - 255

