429669-07-8
 429669-07-8 结构式
429669-07-8 结构式
基本信息
1-BOC-3-甲氧基氮杂环丁烷
3-甲氧基吖丁啶-1-羧酸叔丁酯
3-甲氧基-N-BOC-氮杂环丁烷
1-BOC-3-甲氧基基氮杂环丁烷
1-Boc-3-(methoxy)
N-Boc-3-Methoxyazetidine
1-BOC-3-(METHOXY)AZETIDINE
3-Methoxyazetidine, N-BOC protected
tert-butyl 3-Methoxyazetidine-1-carboxylate
3-Methoxy-azetidine-1-carboxylic acid tert-butyl ester
1-Azetidinecarboxylicacid, 3-Methoxy-, 1,1-diMethylethyl ester
1-Azetidinecarboxylicacid, 3-methoxy-, 1,1-dimethylethyl est...
1-Azetidinecarboxylicacid,3-methoxy-,1,1-dimethylethylester(9CI)
物理化学性质
制备方法
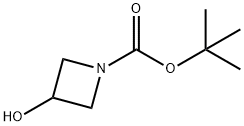
141699-55-0
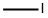
74-88-4

429669-07-8
向3-羟基-氮杂环丁烷-1-羧酸叔丁酯(0.8 g,4.62 mmol)的四氢呋喃(THF,50 mL)溶液中加入氢化钠(60%分散于矿物油中,0.74 g,18.5 mmol)。将反应混合物在0℃下搅拌0.5小时,随后缓慢加入碘甲烷(2.8 mL,46.2 mmol)。反应混合物在室温下搅拌过夜。反应完成后,用水(50 mL)淬灭,并用乙酸乙酯(EtOAc,30 mL×3)萃取。合并有机相,用无水硫酸钠(Na2SO4)干燥,过滤后减压浓缩,得到目标产物3-甲氧基-N-Boc-氮杂环丁烷(0.8 g,收率93%),为油状物。1H NMR(400 MHz,CDCl3):δ 4.11-4.13(m,1H),4.04-4.08(m,2H),3.79-3.83(m,2H),3.27(s,3H),1.43(s,9H)。
参考文献:
[1] Organic Letters, 2014, vol. 16, # 3, p. 856 - 859
[2] Patent: WO2013/53690, 2013, A1. Location in patent: Page/Page column 37; 38
[3] Patent: US2004/14962, 2004, A1. Location in patent: Page/Page column 135
[4] Patent: US2008/214815, 2008, A1. Location in patent: Page/Page column 16
[5] Patent: WO2011/63502, 2011, A1. Location in patent: Page/Page column 50
