181269-69-2
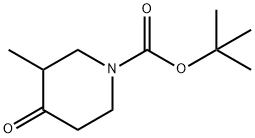 181269-69-2 结构式
181269-69-2 结构式
基本信息
N-BOC-3-甲基-4-哌啶酮
1-BOC-3-甲基-4-哌啶酮
1-叔丁羰基-4-甲基-3-哌啶酮
3-甲基-4-氧哌啶-1-羧酸叔丁酯
3-甲基-4-氧代哌啶-1-甲酸叔丁酯
3-甲基-4-氧代哌啶-1-羧酸叔丁酯
雄甾-1,4,6-三烯-3,17-二酮(Z)
N-BOC-3-甲基-4-哌啶酮(CAS号:181269-69-2)
N-Boc-3-methyl-4-piperidone
1-BOC-3-METHYL-PIPERIDIN-4-ONE
N-BOC-3-Methyl-4-oxopiperidine
1-Boc-4-oxo-3-methyl-piperidine
N-TERT-BUTOXYCARBONYL-3-METHYL-4-PIPERIDONE
tert-Butyl 3-methyl-4-oxopiperidine-1-carboxylate
tert-Butyl 3-methyl-4-oxo-1-piperidinecarboxylate
(R,S)-tert-Butyl 3-methyl-4-oxopiperidine-1-carboxylate
3-Methyl-4-oxo-piperidine-1-carboxylicacidtert-butylester
物理化学性质
制备方法

34737-89-8

24424-99-5

181269-69-2
以1-苄基-3-甲基-哌啶-4-酮(化合物28,15.0 g,0.0738 mol)和二碳酸二叔丁酯(17.7 g,0.0812 mol)为原料,合成N-Boc-3-甲基-哌啶-4-酮(产物27B)的一般步骤如下:将化合物28和二碳酸二叔丁酯溶解于乙酸乙酯(EtOAc,400 mL)中。向溶液中加入氢氧化钯催化剂(4 g),将混合物置于Paar振荡器中,在40 psi的氢气压力下振荡反应24小时。反应完成后,将混合物通过硅藻土过滤,并用乙酸乙酯洗涤硅藻土。合并滤液,减压浓缩,得到无色油状产物27B(15.73 g,0.0738 mol,产率100%)。质谱分析(ESI+,M-tBOC):m/z 114。
参考文献:
[1] Patent: US2005/182095, 2005, A1. Location in patent: Page/Page column 30
[2] Patent: WO2011/159852, 2011, A1. Location in patent: Page/Page column 11-12
[3] Patent: US2010/113465, 2010, A1. Location in patent: Page/Page column 34-35
[4] Patent: WO2018/5881, 2018, A1. Location in patent: Page/Page column 126
[5] Patent: US2010/204274, 2010, A1. Location in patent: Page/Page column 54-55
