53990-33-3
 53990-33-3 结构式
53990-33-3 结构式
基本信息
Cbz-L-苯甘氨酸
CBZ-L-PHENYLGLYCINE
CBZ-PHG-OH
N-ALPHA-CARBOBENZOXY-L-PHENYLGLYCINE
N-ALPHA-CBZ-L-PHENYLGLYCINE
N-CBZ-L-PHENYLGLYCINE
Z-L-PHENYLGLYCINE
Z-PHENYLGLYCINE
Z-(PHENYL)GLY-OH
Z-PHG-OH
Cbz-L-Alpha-Phenylglycine
N-CBZ-L-PHENYLGLYCINE (Z-PHG-OH)
N-CBZ-L-PHENYLGLYCINE(CBZ-PHG-OH)
NALPHA-Benzyloxycarbonyl-L-phenylglycine
BENZYLOXYCARBONYL-L-PHENYLGLYCINE(N-CBZ-L-PHENYLGLYCINE)
Z-L-Phg-OH
N-Carbobenzyloxy-L-phenylglycine
物理化学性质
制备方法

2935-35-5
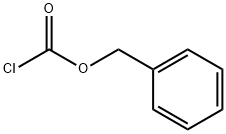
501-53-1
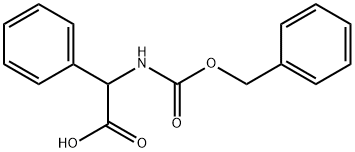
5491-18-9
以L-苯甘氨酸和氯甲酸苄酯为原料合成化合物(CAS:5491-18-9)的一般步骤:向含有2-氨基-2-苯基乙酸(20g,0.12mol)的水溶液(500mL)中,在室温下加入碳酸钠(27.97g,0.264mol)和碳酸氢钠(11.1g,0.132mol)。搅拌混合物直至形成澄清溶液。随后加入丙酮(40mL),并将得到的轻微混浊溶液在冰水浴中冷却至15-20℃。在搅拌下缓慢加入氯甲酸苄酯(Cbz-Cl,28.15g,0.165mol),然后将反应混合物逐渐升温至室温。继续搅拌3小时后,用甲基叔丁基醚(100mL)萃取反应混合物。用盐酸水溶液调节水层的pH至2。将形成的油状物用乙酸乙酯(100mL×2)萃取。合并有机层,用水洗涤后,在真空下浓缩,得到(S)-2-(((苄氧基)羰基)氨基)-2-苯基乙酸(30.2g,收率80%)为白色固体。
参考文献:
[1] Tetrahedron Letters, 2006, vol. 47, # 36, p. 6393 - 6396
[2] Patent: WO2018/136647, 2018, A2. Location in patent: Page/Page column 29-30
[3] Tetrahedron Letters, 1993, vol. 34, # 39, p. 6329 - 6332
[4] Patent: WO2014/210436, 2014, A2. Location in patent: Paragraph 00520; 00521
[5] Patent: EP1076563, 2005, B1. Location in patent: Page/Page column 9; 13
