53995-82-7
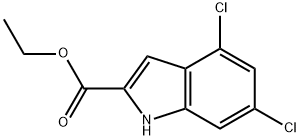 53995-82-7 结构式
53995-82-7 结构式
基本信息
4,6-DICHLOROINDOLE-2-CARBOXYLIC ACID ETHYL ESTER
ETHYL 4,6-DICHLORO-1H-INDOLE-2-CARBOXYLATE
ETHYL 4,6-DICHLOROINDOLE-2-CARBOXYLATE
ETHYL,4,6-DICLOROINDOLE-2-CARBOXYLATE
物理化学性质
制备方法
![2-[2-(3,5-二氯苯基)肼-1-亚基]丙酸乙酯](/CAS/20210111/GIF/103855-01-2.gif)
103855-01-2

53995-82-7
步骤3:中间体4,6-二氯-1H-吲哚-2-羧酸乙酯(I-ic)的制备 将2-[2-(3,5-二氯苯基)肼-1-亚基]丙酸乙酯(26g,94.50mmol,分两批次)与多磷酸(260g,每批)在110℃下搅拌反应3小时。反应完成后,将反应混合物缓慢倒入碎冰中,充分搅拌后过滤收集沉淀。所得固体用饱和NaHCO3水溶液(调节pH至10)碱化,随后用乙酸乙酯(3×150mL)萃取。合并有机层,用盐水洗涤,无水Na2SO4干燥,浓缩。粗产物通过硅胶柱色谱法纯化,使用30%乙酸乙酯的己烷溶液作为洗脱剂,得到4,6-二氯-1H-吲哚-2-羧酸乙酯(I-ic)36g,收率73.8%,为灰白色固体。 1H NMR (400 MHz, DMSO-d6): δ 12.46 (s, 1H), 7.46 (s, 1H), 7.30 (s, 1H), 7.12 (s, 1H), 4.36 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H)。 ESI MS: m/z 255.8 (M-H)。
参考文献:
[1] Patent: WO2006/109633, 2006, A1. Location in patent: Page/Page column 182-183
[2] Organic Process Research and Development, 2000, vol. 4, # 6, p. 477 - 487
[3] ACS Medicinal Chemistry Letters, 2014, vol. 5, # 4, p. 326 - 330
[4] Journal of Labelled Compounds and Radiopharmaceuticals, 2011, vol. 54, # 10, p. 645 - 656
[5] ACS Infectious Diseases, 2017, vol. 3, # 3, p. 225 - 236
