55954-23-9
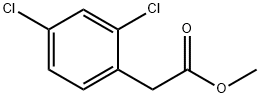 55954-23-9 结构式
55954-23-9 结构式
基本信息
2,4-二氯苯乙酸甲酯
METHYL 2,4-DICHLOROPHENYLACETATE
METHYL 2,4-DICHLOROPHENYLACETATE, 100MG, NEAT
METHYL-2,4-DICHLOROPHENYLACETATE,1X1ML,A CETONE 100UG/ML
2,4-dcaa methyl ester
47339
methyl 2,4-dichlorophenylacetate solution
2,4-DICHLOROPHENYLACETIC ACID METHYL ESTER STANDARD
物理化学性质
制备方法
67-56-1

19719-28-9

55954-23-9
以甲醇和2,4-二氯苯乙酸为原料合成2,4-二氯苯基乙酸甲酯的一般步骤:向2,4-二氯苯乙酸(3.5g,17.1mmol)的甲醇(100mL)溶液中缓慢加入浓硫酸(20滴)。将反应混合物加热回流12小时。反应完成后,通过旋转蒸发仪在减压下除去溶剂。将残余物用冷水(50mL)稀释,并用乙酸乙酯(3×50mL)萃取。合并有机层,依次用饱和碳酸氢钠溶液(50mL)和饱和食盐水(50mL)洗涤,然后用无水硫酸钠干燥并过滤。再次通过旋转蒸发仪在减压下除去溶剂,得到粗产物。粗产物通过硅胶柱色谱法(洗脱剂比例为4:1的己烷/乙酸乙酯)纯化,得到2,4-二氯苯基乙酸甲酯(3.56g,收率95%)为透明油状物。产物结构经1H NMR(DMSO-d6)确认:δ7.40(s,1H),7.22(d,J=1.5Hz,2H),3.74(s,2H),3.71(s,3H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2003, vol. 11, # 13, p. 2843 - 2866
[2] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 1, p. 245 - 249
[3] Patent: US2016/31892, 2016, A1. Location in patent: Paragraph 0195-0196
[4] ACS Medicinal Chemistry Letters, 2010, vol. 1, # 4, p. 145 - 149
[5] Bulletin of the Korean Chemical Society, 2010, vol. 31, # 8, p. 2315 - 2321
