5622-36-6
 5622-36-6 结构式
5622-36-6 结构式
基本信息
6-喹啉乙酸甲酯
喹啉-6-乙酸甲酯
METHYL 6-QUINOLINEACETATE
methyl quinolin-6-ylacetate
Methyl 2-quinolin-6-ylacetate
Methyl 6-quinolineacetate ,95%
6-Quinolineacetic acid methyl ester
Quinolin-6-Yl-Acetic Acid Methyl Ester
物理化学性质
制备方法
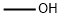
67-56-1

5622-34-4

5622-36-6
实施例1. 喹啉-6-乙酸甲酯的制备:如方案1的步骤i所示,将浓硫酸(206 mL,3.868 mol)缓慢滴加到溶解于6.5 L甲醇中的6-喹啉乙酸(化合物1001,658.2 g,3.516 mol,Okeanos Tech Co.,目录号OK-J-05024)溶液中。滴加过程中观察到轻微放热。滴加完毕后,将反应混合物在回流条件下搅拌4小时。反应完成后,冷却至室温,减压蒸馏除去挥发性溶剂。残余物用4 L乙酸乙酯稀释,并在冰浴中冷却。随后,用2 N NaOH溶液(2.1 L,1.2当量)调节pH至4,再用饱和碳酸氢钠溶液调节pH至8。分离有机层和水层,水层用乙酸乙酯萃取两次。合并的有机相依次用饱和碳酸氢钠溶液、水和盐水洗涤,然后用无水MgSO4干燥。过滤后,减压蒸发溶剂,得到喹啉-6-乙酸甲酯(化合物1002,696.8 g,收率98%)为透明棕色油状物:ESMS (M + 1), 202.14; 1H NMR (300.0 MHz, DMSO-d6) δ 8.90 (1H, dd, J = 1.7, 4.2 Hz), 8.14-8.10 (1H, m), 8.08 (1H, d, J = 8.7 Hz), 7.72 (1H, d, J = 1.4 Hz), 7.65 (1H, dd, J = 2.0, 8.7 Hz), 7.40 (1H, dd, J = 4.2, 8.3 Hz), 3.83 (2H, s), 3.73 (3H, s)。
参考文献:
[1] Patent: WO2010/59668, 2010, A1. Location in patent: Page/Page column 20-21
[2] Patent: WO2016/8011, 2016, A1. Location in patent: Page/Page column 67
[3] Patent: WO2013/19682, 2013, A1. Location in patent: Page/Page column 117
[4] Patent: WO2008/144767, 2008, A1. Location in patent: Page/Page column 110; 115
[5] Journal of Medicinal Chemistry, 2013, vol. 56, # 17, p. 6651 - 6665
