6404-31-5
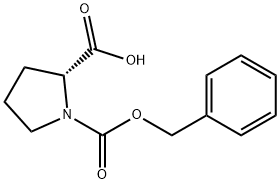 6404-31-5 结构式
6404-31-5 结构式
基本信息
N-苄氧羰基-D-脯氨酸
CBZ-D-脯氨酸
苄氧羰基-D-脯氨酸
N-苄氧羰酰基-D-脯氨酸
N-苄氧羰基-D-脯氨酸, 98+%
1,2-PYRROLIDINEDICARBOXYLIC ACID, 1-(PHENYLMETHYL) ESTER, (R)-
1-[(BENZYLOXY)CARBONYL]PYRROLIDINE-2-CARBOXYLIC ACID
BENZYLOXYCARBONYL-D-PROLINE
(+)-CARBOBENZYLOXY-D-PROLINE
CBZ-D-PROLINE
CBZ-D-PRO-OH
D-Z-PRO
N-ALPHA-BENZYLOXYCARBONYL-D-PROLINE
N-ALPHA-CARBOBENZOXY-D-PROLINE
N-ALPHA-CARBOBENZOXY-D-PYRROLIDINE-2-CARBOXYLIC ACID
N-ALPHA-CBZ-D-PROLINE
N-BENZYLOXYCARBONYLAMINO-D-PROLINE
N-BENZYLOXYCARBONYL-D-PROLINE
N-CARBOBENZOXY-D-PROLINE
N-CBZ-D-PROLINE
N-Z-D-PROLINE
(R)-N-BENZYLOXYCARBONYLPROLINE
Z-D-PROLINE
Z-D-PRO-OH
物理化学性质
制备方法
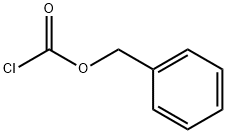
501-53-1

344-25-2

6404-31-5
步骤-1:制备(R)-1-(苄氧基羰基)吡咯烷-2-羧酸(12a)。向D-脯氨酸(1.2 g,10.42 mmol)在2 N NaOH水溶液(20.85 mL,41.7 mmol)中的搅拌溶液中,在0℃下缓慢滴加氯甲酸苄酯(1.488 mL,10.42 mmol)。反应混合物逐渐升温至室温并搅拌过夜。反应完成后,用甲基叔丁基醚(MTBE,2×25 mL)洗涤反应混合物,然后用浓盐酸酸化至pH≈2。用乙酸乙酯(2×200 mL)萃取水相,合并有机相,依次用水(50 mL)和饱和食盐水(25 mL)洗涤。有机层经无水硫酸钠干燥后,减压浓缩,得到白色固体(R)-1-(苄氧基羰基)吡咯烷-2-羧酸(12a)(2.41 g,9.67 mmol),收率93%。产物经1H NMR(300 MHz, DMSO-d6)和质谱(MS)表征:1H NMR δ 12.66(s, 1H), 7.42-7.25(m, 5H), 5.14-4.97(m, 2H), 4.20(ddd, J = 22.7, 8.8, 3.5 Hz, 1H), 3.50-3.25(m, 2H), 2.32-2.08(m, 1H), 1.97-1.75(m, 3H); MS(ES+)m/z 250.2([M+H]+), 272.2([M+Na]+),(ES-)m/z 248.2([M-H]-), 284.2([M+Cl]-), 497.4([2M-H]-)。
参考文献:
[1] Patent: WO2015/62486, 2015, A1. Location in patent: Paragraph 00191
[2] Patent: WO2017/59178, 2017, A1. Location in patent: Page/Page column 73
[3] Journal of Pharmaceutical Sciences, 1991, vol. 80, # 9, p. 837 - 842
[4] Angewandte Chemie - International Edition, 2006, vol. 45, # 28, p. 4593 - 4597
[5] European Journal of Medicinal Chemistry, 2009, vol. 44, # 7, p. 2807 - 2814
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | C1730 | N-苄氧羰基-D-脯氨酸 N-Carbobenzoxy-D-proline | 6404-31-5 | 5G | 140元 |
| 2025/12/22 | XW64043152 | N-苄氧羰基-D-脯氨酸 n-(benzyloxycarbonyl)-d-proline;n-benzyloxycarbonyl-d-proline;n-(carbobenzoxy)-d-proline;z-d-proline;cbz-d-proline;cbz-d-pro-oh;z-d-pro-oh | 6404-31-5 | 5G | 19元 |
| 2025/12/22 | C1730 | N-苄氧羰基-D-脯氨酸 N-Carbobenzoxy-D-proline | 6404-31-5 | 25G | 470元 |
