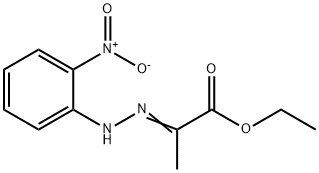
6960-46-9
 6960-46-9 结构式
6960-46-9 结构式
基本信息
7-NITROINDOLE-2-CARBOXYLIC ACID ETHYL ESTER
ETHYL 7-NITRO-1H-INDOLE-2-CARBOXYLATE
ETHYL 7-NITROINDOLE-2-CARBOXYLATE
LABOTEST-BB LT00441290
物理化学性质
制备方法
292853-66-8

6960-46-9
实施例2:7-硝基-1H-吲哚-2-羧酸乙酯的合成 在250 mL三颈烧瓶中,加入干燥的化合物1a和1b(12.00 g,47.76 mmol)及多磷酸(64.56 g,191.05 mmol)。将混合物加热至70℃并充分搅拌,随后升温至80℃反应12小时。通过薄层色谱(TLC)监测反应进程。反应完成后,趁热将粘稠的黑色反应液缓慢倒入冷水(400 g)中,搅拌至多磷酸完全水解。此时,溶液呈黑色,并有棕黑色固体析出。过滤收集固体,干燥。采用Soxhlet萃取法,以石油醚(沸点60-90℃)为溶剂,对干燥后的棕黑色固体进行连续萃取12小时。萃取完成后,浓缩石油醚溶液,得到黄色固体。进一步通过乙醇重结晶,获得针状黄色晶体,即目标产物7-硝基-1H-吲哚-2-羧酸乙酯(10.5 g,收率94%)。 产物表征: 熔点:92-93℃(文献值:91-93℃); 1H NMR (200 MHz, CDCl3) δ: 1.41 (t, J = 7.2 Hz, 3H, CH3), 4.42 (q, J = 7.2 Hz, 2H, CH2), 7.20 (t, J = 7.9 Hz, 1H, ArH), 7.28 (d, J = 2.1 Hz, 1H, ArH), 7.97 (d, J = 7.8 Hz, 1H, ArH), 8.21 (d, J = 8.0 Hz, 1H, ArH), 10.25 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ: 14.8, 62.0, 109.7, 120.5, 122.6, 130.0, 130.6, 131.1, 131.3, 133.8, 161.1; MS (EI) m/z: 235 (M+1, 100%), 234 (M+, 69%), 189 (M-45, 38%)。
参考文献:
[1] Patent: EP2366687, 2011, A2. Location in patent: Page/Page column 8-10
[2] Bioorganic and Medicinal Chemistry, 2014, vol. 22, # 6, p. 1850 - 1862
[3] Patent: CN107098846, 2017, A. Location in patent: Paragraph 2633; 2640-2642
[4] Journal of the American Chemical Society, 1958, vol. 80, p. 4621
[5] Journal of Organic Chemistry, 1957, vol. 22, p. 84
