912556-91-3
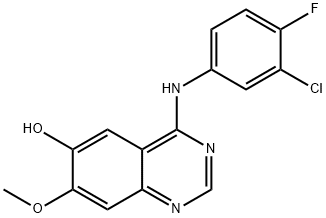
 912556-91-3 结构式
912556-91-3 结构式
基本信息
N-(3-氯-4-氟苯基)-6-(3-氯丙氧基)-7-甲基喹唑啉-4-胺
N-(3-氯-4-氟苯基)-6-(3-氯丙氧基)-7-甲氧基-4-喹唑啉胺
Gefitinib InterMediate B ISO 9001:2015 REACH
N-(3-Chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxy-4-quinazolinamine
N-(3-chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-Methoxyquinazolin-4-aMine
4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxy-
6-(3-chloropropoxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-ylamine
物理化学性质
制备方法

109-70-6
184475-71-6

912556-91-3
以4-((3-氯-4-氟苯基)氨基)-7-甲氧基喹唑啉-6-醇(20.00g)、K2CO3(10.37g)、KI(1.04g)、1-溴-3-氯丙烷(7.50mL)和DMF(150mL)为原料,将混合物在40℃下搅拌反应6小时。反应完成后,将反应混合物倒入水中,过滤收集固体。通过硅胶柱色谱法(洗脱剂:乙酸乙酯)纯化滤渣,得到目标化合物N-(3-氯-4-氟苯基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉-4-胺,为浅黄色液体(22.05g,收率89.00%)。该化合物的结构通过以下光谱数据确认:质谱(ESI,正离子模式)m/z:396.1([M+H]+);1H NMR(400MHz,CDCl3)δ:2.01(m,2H),3.68(t,J=4.2Hz,2H),4.00(s,3H),4.10(t,J=4.2Hz,2H),6.80(s,1H),7.16(s,1H),7.26(s,1H),7.30(s,1H),7.47(s,1H),8.64(s,1H)ppm。
参考文献:
[1] Patent: WO2013/71697, 2013, A1. Location in patent: Paragraph 00197
[2] Patent: US2014/228361, 2014, A1. Location in patent: Paragraph 0277-0278
[3] Patent: US2014/206664, 2014, A1. Location in patent: Paragraph 0225; 0229
[4] Patent: EP2796451, 2014, A1. Location in patent: Paragraph 0082
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0291255691303 | N-(3-氯-4-氟苯基)-6-(3-氯丙氧基)-7-甲基喹唑啉-4-胺 N-(3-Chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxyquinazolin-4-amine | 912556-91-3 | 1g | 2065元 |
| 2025/12/22 | XW0291255691302 | N-(3-氯-4-氟苯基)-6-(3-氯丙氧基)-7-甲基喹唑啉-4-胺 N-(3-Chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxyquinazolin-4-amine | 912556-91-3 | 250mg | 757元 |
| 2025/12/22 | XW0291255691301 | N-(3-氯-4-氟苯基)-6-(3-氯丙氧基)-7-甲基喹唑啉-4-胺 N-(3-Chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxyquinazolin-4-amine | 912556-91-3 | 100mg | 448元 |
