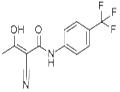
2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide
- CAS No.
- 108605-62-5
- Chemical Name:
- 2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide
- Synonyms
- Su 20;CS-862;Aubagio;Aids013145;Aids-013145;rac-A77 1626;Teriflunomide;(E/Z)-Teriflunomide;TeriflunoMide, A77 1726;Teriflunomide((E/Z) Mixture)
- CBNumber:
- CB6464433
- Molecular Formula:
- C12H9F3N2O2
- Molecular Weight:
- 270.21
- MDL Number:
- MFCD00910058
- MOL File:
- 108605-62-5.mol
| Product description | Number | Pack Size | Price |
| Leflunomide Related Compound B pharmaceutical secondary standard, certified reference material | PHR2878 | 50MG | $619.4 |
| Leflunomide Related Compound B United States Pharmacopeia (USP) Reference Standard | 1357056 | 20mg | $1330 |
| Teriflunomide ≥99%(HPLC) | 5069 | 50 | $151 |
| A771726(E/Z)Mixture | A771720 | 50mg | $80 |
| Teriflunomide | 256904 | 50mg | $389 |
| More product size | |||
| Melting point | 229-232°C |
|---|---|
| Boiling point | 410.8±45.0 °C(Predicted) |
| Density | 1.424±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO (up to 30 mg/ml), or in Ethanol (up to 5 mg/ml with warming). |
| pka | 5.20±0.50(Predicted) |
| form | Solid |
| color | White |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| Major Application | pharmaceutical |
| InChI | 1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- |
| InChIKey | UTNUDOFZCWSZMS-YFHOEESVSA-N |
| SMILES | O=C(/C(C#N)=C(O)/C)NC1=CC=C(C(F)(F)F)C=C1 |
| FDA UNII | 1C058IKG3B |
| ATC code | L04AA31 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301 |
| Precautionary statements | P264-P270-P301+P310-P405-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| WGK Germany | WGK 3 |
| HS Code | 2926900005 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral |
| Toxicity | mouse,LD50,intraperitoneal,100mg/kg (100mg/kg),United States Patent Document. Vol. #5494911, |
2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2878 | Leflunomide Related Compound B pharmaceutical secondary standard, certified reference material | 108605-62-5 | 50MG | $619.4 | 2025-07-31 | Buy |
| Sigma-Aldrich | 1357056 | Leflunomide Related Compound B United States Pharmacopeia (USP) Reference Standard | 108605-62-5 | 20mg | $1330 | 2025-07-31 | Buy |
| Tocris | 5069 | Teriflunomide ≥99%(HPLC) | 108605-62-5 | 50 | $151 | 2021-12-16 | Buy |
| TRC | A771720 | A771726(E/Z)Mixture | 108605-62-5 | 50mg | $80 | 2021-12-16 | Buy |
| Usbiological | 256904 | Teriflunomide | 108605-62-5 | 50mg | $389 | 2021-12-16 | Buy |
2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide Chemical Properties,Uses,Production
Description
Teriflunomide (Aubagio®), also known as A77 1726, is an immunosupressant marketed by Sanofi for the teatment of multiple sclerosis (MS). Teriflunomide is the active metabolite of leflunomide, used for treatment of patients diagnosed with rheumatoid arthritis, and therefore simultaneously can be used as a treatment for rheumatoid arthritis.Teriflunomide acts as an inhibitor of the mitochondrial enzyme dihydrorotate dehydrogenase, inhibiting pyrimidine formation, and resulting in reduced B and T cell proliferation.
Chemical Properties
White Solid
Uses
The active metabolite of Leflunomide, 2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide can be used as a potent disease-modifying antirheumatic drug used in the treatment of rheumatoid arthritis.
Definition
ChEBI: Teriflunomide is an enamide obtained by formal condensation of the carboxy group of (2Z)-2-cyano-3-hydroxybut-2-enoic acid with the anilino group of 4-(trifluoromethyl)aniline. Used for the treatment of relapsing forms of multiple sclerosis and rheumatoid arthritis. It has a role as an EC 1.3.98.1 [dihydroorotate oxidase (fumarate)] inhibitor, a tyrosine kinase inhibitor, a hepatotoxic agent, a drug metabolite and a non-steroidal anti-inflammatory drug. It is a nitrile, an enol, an aromatic amide, an enamide, a member of (trifluoromethyl)benzenes and a secondary carboxamide.
Clinical Use
Immunomodulating agent:
Treatment of relapsing remitting multiple sclerosis
Synthesis
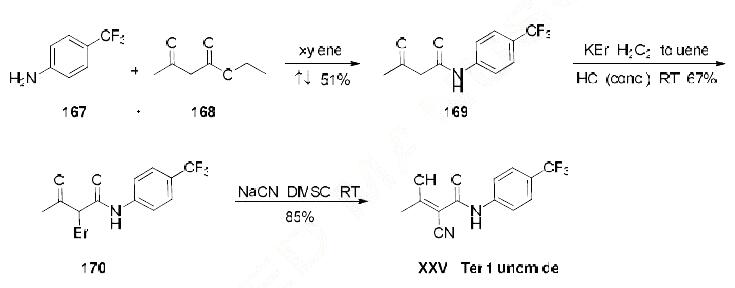
Numerous syntheses of teriflunomide have been developed to date, most relying on the use of 4- trifluoromethyl aniline (167). The current optimized method for scale-up synthesis of teriflunomide, developed by Keshav and coworkers, begins with reaction of commercial 4-trifluoromethyl aniline 167 and ethylacetoacetate (168) in refluxing xylenes, providing acetoamidate 169 in 51% yield . The resulting acetoamidate 169 was then treated with H2O2, KBr, and concentrated HCl at room temperature, providing bromide 170 in 67% yield. Bromide 170 was reacted with NaCN in DMSO, generating teriflunomide (XXVI) in 85% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Lipid-lowering agents: effect significantly reduced by
colestyramine - avoid; concentration of rosuvastatin
increased - consider reducing rosuvastatin dose.
Live vaccines: risk of generalised infections - avoid.
Metabolism
Teriflunomide is the active metabolite of leflunomide. It is moderately metabolised and teriflunomide is the only component detected in plasma. The main biotransformation pathway is hydrolysis with oxidation being a minor pathway. Secondary pathways involve oxidation, N-acetylation and sulfate conjugation. Teriflunomide is excreted in the gastrointestinal tract mainly through the bile as unchanged drug and most likely by direct secretion.
storage
Store at +4°C
References
[1] S. MANNA B A. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression.[J]. Journal of immunology, 1999, 162 4 1: 2095-2102. DOI:10.4049/jimmunol.162.4.2095
[2] JUNE P. DAVIS. The Immunosuppressive Metabolite of Leflunomide Is a Potent Inhibitor of Human Dihydroorotate Dehydrogenase[J]. Biochemistry Biochemistry, 1996, 35 4: 1270-1273. DOI:10.1021/bi952168g
[3] QUEE MING SEAH. Oxidative bioactivation and toxicity of leflunomide in immortalized human hepatocytes and kinetics of the non-enzymatic conversion to its major metabolite, A77 1726.[J]. Drug metabolism letters, 2008, 2 3: 153-157. DOI:10.2174/187231208785425791
2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718; +8613336195806 | sales@capot.com | China | 29735 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21599 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29833 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354; +17819995354 | marketing@targetmol.com | United States | 32470 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2251 | 58 |
| RongNa Biotechnology Co.,Ltd | +86-86-13583358881 +8618560316533 | Brad@rongnabiotech.com | China | 3388 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 19781 | 58 |
View Lastest Price from 2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2026-01-27 | A77 1726
108605-62-5
|
0.99 | RongNa Biotechnology Co.,Ltd | ||||
 |
2026-01-24 | (E/Z)-Teriflunomide
108605-62-5
|
US $48.00 / mg | 99.76% | 10g | TargetMol Chemicals Inc. | ||
 |
2019-07-06 | A77 1726
108605-62-5
|
US $1.00 / KG | 1G | 99% | 100KG | Career Henan Chemical Co |
-

- A77 1726
108605-62-5
- 0.99
- RongNa Biotechnology Co.,Ltd
-

- (E/Z)-Teriflunomide
108605-62-5
- US $48.00 / mg
- 99.76%
- TargetMol Chemicals Inc.
-

- A77 1726
108605-62-5
- US $1.00 / KG
- 99%
- Career Henan Chemical Co
108605-62-5(2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide)Related Search:
1of4





