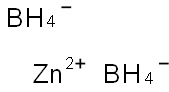아연보로하이드라이드
|
|
아연보로하이드라이드 속성
- InChI
- InChI=1S/2BH4.Zn/h2*1H4;/q2*-1;+2
- InChIKey
- PTJGRTOJBSRNJP-UHFFFAOYSA-N
- SMILES
- [Zn+2].[BH4-].[BH4-]
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 그림문자(GHS): |
 
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||||||
| 예방조치문구: |
|
아연보로하이드라이드 C화학적 특성, 용도, 생산
화학적 성질
Zinc borohydride ether solution is sensitive to moisture and should be stored and used under the protection of inert gas.용도
zinc borohydride is unique because of the better coordination ability of Zn2+, which imparts selectivity in hydridetransferring reactions. Zinc borohydride is moderately stable in ethereal solution and has many applications in organic synthesis. Zinc borohydride, as a nonconventional hydride transferring agent, has been reported as an efficient chemo-, regio- and stereoselective reducing agent in several complex substrates. It can be used in a range of aprotic solvents such as, THF, Et2O and DME. Several combination reducing systems of Zn(BH4)2 such as Zn(BH4)2/TMEDA, Zn(BH4)2/Me3SiCl, Zn(BH4)2/ TFA/DME, Zn(BH4 ) 2 /H2O, 1Zn(BH4 ) 2 /Al 2O3 , and Zn(BH4)2/C are interesting and have been used for different reduction purposes.제조 방법
Zinc borohydride (Zn(BH4)2) needs to be prepared by in situ reaction, anhydrous zinc chloride is reacted with sodium borohydride in anhydrous ether solvent.화학 반응
Zinc borohydride is often used as a reducing agent in organic synthesis, and its reducing ability is comparable to that of lithium aluminum hydride, and its selectivity is good. It can selectively reduce aldehydes, ketones, carboxylic acids and their derivatives, imines, nitriles, epoxy compounds, alkenes (alkynes), etc., and has good stereoselectivity for chiral molecules. Because the ether solution of zinc borohydride is neutral, it is very suitable for substrates containing base-sensitive functional groups such as cyano, ester, γ-lactone, etc. In addition, when saturated ketones and α,β-unsaturated ketones coexist, the reduction of saturated ketones can be preferentially achieved selectively.
Reduction Reaction Zinc Borohydride
아연보로하이드라이드 준비 용품 및 원자재
원자재
준비 용품
아연보로하이드라이드 공급 업체
글로벌( 66)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Cangzhou Lingang Xingchen Chemical Co.,Ltd. | +8613820956817 |
sales1@daofuchem.com | China | 6 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +8613288715578 |
sales@hbmojin.com | China | 12248 | 58 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1001 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9636 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23541 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 49977 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 |
product@acmec-e.com | China | 33338 | 58 |
아연보로하이드라이드 관련 검색:
아연에틸페닐디티오카바메이트 아연비스[p-톨루엔설피네이트] 아연피테이트 황화아연(입방형)
Zinc dibenzyldithiocarbamate
Zinc disodium EDTA
ZINC OXALATE
Zinc borate
Zinc benzenesulfinate dihydrate
Zinc chloride
ZINC CARBONATE BASIC
Zinc hydroxide
Zinc ammonium chloride
Zinc acetate
Zinc fluoride
Zinc sulfate heptahydrate
Bacitracin zinc
Zinc acrylate