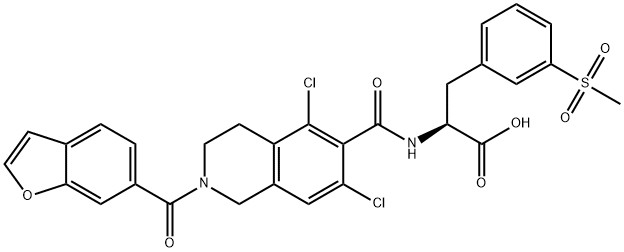
lifitegrast
|
|
lifitegrast 속성
- 녹는점
- >163°C (dec.)
- 끓는 점
- 811.9±65.0 °C(Predicted)
- 밀도
- 1.479±0.06 g/cm3(Predicted)
- 저장 조건
- Hygroscopic, -20°C Freezer, Under inert atmosphere
- 용해도
- DMSO(약간, 가열, 초음파 처리), 메탄올(약간, 초음파 처리)에 용해됨
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 3.14±0.10(Predicted)
- 색상
- 흰색에서 황백색까지
- 안정성
- 흡습성
- InChIKey
- JFOZKMSJYSPYLN-QHCPKHFHSA-N
- SMILES
- C(O)(=O)[C@H](CC1=CC=CC(S(C)(=O)=O)=C1)NC(C1C(Cl)=CC2=C(C=1Cl)CCN(C(C1C=C3OC=CC3=CC=1)=O)C2)=O
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 그림문자(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||||||||||||||||
| 예방조치문구: |
|
lifitegrast C화학적 특성, 용도, 생산
개요
Lifitegrast (Xiidra; lifitegrast sodium; SAR1118; SHP606; SPD606) is a lymphocyte functionassociated antigen-1 (LFA-1) antagonist. It inhibits T-cell inflammation by blocking the binding of two key surface proteins (LFA-1 and ICAM-1) that mediate the chronic inflammatory cascade associated with dry eye disease. In a phase III clinical trial, lifitegrast 5% ophthalmic solution (50 mg/mL) is administered as a single 0.2mL eye drop twice a day into each eye for an 84 day treatment period.
Lifitegrast does not currently have Marketing Authorisation in the EU for any indication. Lifitegrast is licensed for use in the USA for treatment of the signs and symptoms of dry eye disease. The most common adverse reactions (incidence 5-25%) reported following the use of lifitegrast are instillation site irritation, dysgeusia, and decreased visual acuity.
화학적 성질
Lifitegrast is a white to off-white powder which is soluble in water.용도
Lifitegrast is used for the treatment of signs and symptons of dry eye diseases. It also Inhibites corneal inflammation that is capable of causing pains, blurred vision and ocular discomfort in sufferer.Indications
Topical lifitegrast was approved by the FDA for the treatment of dry eye. Lifitegrast decreases inflammation by blocking the interaction between intercellular adhesion molcule 1 and lymphocyte function- -associated antigen 1. In four, large, multicellular, randomized clinical trials, lifitegrast was shown to be effective in improving the signs and symptoms of dry eye. The side effects of lifitegrast include transient ocular irritation and dysgeusia. Further studies are needed to explore the effectiveness of combination therapy such as the concomitant use of topical cyclosporine and topical liftegrast.정의
ChEBI: Lifitegrast is an N-acyl-L-alpha-amino acid obtained by formal condensation of the carboxy group of N-[2-(1-benzofuran-6-carbonyl)]-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid with the amino group of 3-(methanesulfonyl)-L-phenylalanine. Used for treatment of keratoconjunctivitis sicca (dry eye syndrome). It has a role as an anti-inflammatory drug and a lymphocyte function-associated antigen-1 antagonist. It is a L-phenylalanine derivative, a sulfone, a N-acyl-L-alpha-amino acid, a member of isoquinolines and a member of 1-benzofurans.Mechanism of action
Lifitegrast binds to the integrin lymphocyte function-associated antigen-1 (LFA-1), a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1). ICAM-1 may be overexpressed in corneal and conjunctival tissues in dry eye disease. LFA-1/ICAM-1 interaction can contribute to the formation of an immunological synapse resulting in T-cell activation and migration to target tissues. In vitro studies demonstrated that lifitegrast may inhibit T-cell adhesion to ICAM-1 in a human T-cell line and may inhibit secretion of inflammatory cytokines in human peripheral blood mononuclear cells. The exact mechanism of action of lifitegrast in dry eye disease is not known.Pharmacokinetics
In a subset of dry eye disease patients (n=47) enrolled in a Phase 3 trial, the pre-dose (trough) plasma concentrations of lifitegrast were measured after 180 and 360 days of topical ocular dosing (1 drop twice daily) with Xiidra (lifitegrast ophthalmic solution) 5%. A total of nine (9) of the 47 patients (19%) had plasma lifitegrast trough concentrations above 0.5 ng/mL (the lower limit of assay quantitation). Trough plasma concentrations that could be quantitated ranged from 0.55 ng/mL to 3.74 ng/mL.부작용
A multicenter, randomized, double-masked, placebo-controlled phase 3 study (n = 331) evaluating the safety of lifitegrast ophthalmic solution for the treatment of dry eye disease reported the most common non-ocular effect was dysgeusia (change in taste) occurring in 16.4% of patients in the lifitegrast group and 1.8% of the placebo group.참고 문헌
[1] Stacy L. Haber. “Lifitegrast: a novel drug for patients with dry eye disease.” ACS Applied Bio Materials (2019).lifitegrast 준비 용품 및 원자재
원자재
5,7-디클로로-2-트리틸-1,2,3,4-테트라히드로이소퀴놀린-6-카르복실산
benzyl (S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoate
(S)-2-(tert-부톡시카르보닐아미노)-3-(3-(메틸술포닐)페닐)프로판산
3,5-디클로로벤즈알데히드
lifitegrast impurity C
벤조푸란-6-카르복실산
준비 용품
lifitegrast 공급 업체
글로벌( 291)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| PharmOrgSyn Laboratories Co., Ltd. | +86-021-38228826 +86-13916602830 |
sales@pharmorgsyn.cn | China | 139 | 58 |
| Protheragen-ING | +16313385890 |
info@protheragen-ing.com | United States | 3868 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 |
sales@sdperfect.com | China | 294 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 |
sales@sjar-tech.com | China | 504 | 58 |
| Wuhan JiyunZen Tech Co., Ltd. | +86-18062099985 |
Amyjiyunzen@yeah.net | China | 663 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21620 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29847 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 10405 | 58 |
lifitegrast 관련 검색:
6-이소퀴놀린카르복실산,5,7-디클로로-1,2,3,4-테트라히드로-,염산염(1:1) L-페닐알라닌,N-[(5,7-디클로로-1,2,3,4-테트라히드로-6-이소퀴놀리닐)카르보닐]-3-(메틸술포닐)-,페닐메틸에스테르,염산염(1:1) 2-(tert-부톡시카르보닐)-5,7-디클로로-1,2,3,4-테트라히드로이소퀴놀린-6-카르복실산 벤질(S)-2-아미노-3-(3-(메틸술포닐)페닐)프로파노에이트 벤조푸란-6-카르복실산 (S)-2-아미노-3-(3-(메틸술포닐)페닐)프로판산 메틸(S)-2-아미노-3-(3-(메틸설포닐)페닐)프로파노에이트염산염 메틸5,7-디클로로-1,2,3,4-테트라히드로이소퀴놀린-6-카르복실레이트 벤질(S)-2-아미노-3-(3-(메틸술포닐)페닐)프로파노에이트 (S)-2-(tert-부톡시카르보닐아미노)-3-(3-(메틸술포닐)페닐)프로판산
Ethyl benzofuran-6-carboxylate
(R)-Benzyl 2-amino-3-(3-(methylsulfonyl)phenyl)propanoate hydrochloride
benzyl (S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoate
2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester
Carbomer
Capecitabine
Linsitinib
Linagliptin