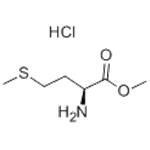
- L-Met-OMe.HCl
-
- $0.00 / 1kg
-
2025-09-19
- CAS:2491-18-1
- Min. Order: 100kg
- Purity: 98%
- Supply Ability: 1T+
|
| | L-Methionine methyl ester hydrochloride Basic information |
| | L-Methionine methyl ester hydrochloride Chemical Properties |
| Melting point | 151-153 °C(lit.) | | alpha | 26 º (c=5, H2O 24 ºC) | | refractive index | 26 ° (C=1, H2O) | | storage temp. | Inert atmosphere,2-8°C | | solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | | form | Powder | | color | White to beige | | Optical Rotation | [α]22/D +25.9°, c = 1 in H2O | | Water Solubility | Soluble in water. | | Sensitive | Hygroscopic | | BRN | 3913214 | | InChI | InChI=1/C6H13NO2S.ClH/c1-9-6(8)5(7)3-4-10-2;/h5H,3-4,7H2,1-2H3;1H/t5-;/s3 | | InChIKey | MEVUPUNLVKELNV-USHJBNIQNA-N | | SMILES | [C@H](N)(CCSC)C(=O)OC.Cl |&1:0,r| | | CAS DataBase Reference | 2491-18-1(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 24/25-26 | | WGK Germany | 3 | | HS Code | 29309090 |
| | L-Methionine methyl ester hydrochloride Usage And Synthesis |
| Chemical Properties | Crystalline | | Uses | L-Methionine methyl ester hydrochloride is used as a starting material for the synthesis of a protected glycine derivative, a versatile asymmetric building block. | | reaction suitability | reaction type: solution phase peptide synthesis | | Synthesis | Synthesis Example 1: L-methionine (0.5 g, 1 eq.) was suspended in a 100 mL round-bottomed flask fitted with a magnetic stirrer, methanol (30 mL, concentration 0.1 M) was added and cooled to 0°C under nitrogen atmosphere. Thionyl chloride (0.5 mL, 2 equiv.) was added slowly and dropwise to the above suspension, followed by heating and refluxing the reaction for 16 hours. After completion of the reaction, the solvent was removed by rotary evaporator to give a light yellow solid. The solid was ground with hot ether and the ether solution was removed by filtration to give a white solid L-methionine methyl ester hydrochloride (0.65561 g, 98% yield). The product was further purified by vacuum drying.1H NMR (D2O, 500 MHz) δH: 4.24 (1H, m, CH), 3.79 (3H, s, OCH3), 2.63 (2H, t, CH2), 2.25 (1H, m, CH2), 2.16 (1H, m, CH2), 2.06 (1H, s, SCH3). 13C NMR (D2O, 125 MHz) δC: 170.58 (C=O), 53.64 (OCH3), 51.70 (CH), 28.71 (CH2), 28.41 (CH2), 13.85 (SCH3). IR (neat, vmax, cm-1): 2880.8/2676.2 (CH3, CH2, CH), 2016.2, 1742.2 (C=O, ester), 1483.6, 1443.5, 1227, 1194.7, 1149.8, 1079.5 (C-O). HRMS m/z (+ESI): C6H14NO2S, calculated mass = 164.074 (error = 0.07 ppm). | | References | [1] Journal of Organic Chemistry, 2011, vol. 76, # 1, p. 201 - 215
[2] Journal of the American Chemical Society, 2017, vol. 139, # 40, p. 14077 - 14089
[3] Patent: WO2012/131313, 2012, A1. Location in patent: Page/Page column 49
[4] Patent: US2014/39200, 2014, A1. Location in patent: Paragraph 0376-0380
[5] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2006, vol. 45, # 8, p. 1942 - 1944 |
| | L-Methionine methyl ester hydrochloride Preparation Products And Raw materials |
|