LIDOFLAZINE, 98.5% manufacturers
- Lidoflazine
-

- $0.00 / 1kg
-
2026-01-16
- CAS:3416-26-0
- Min. Order: 1kg
- Purity: 98% up by HPLC
- Supply Ability: 20tons
- Lidoflazine
-

- $1840.00 / 50mg
-
2026-01-05
- CAS:3416-26-0
- Min. Order:
- Purity:
- Supply Ability: 10g
- Lidoflazine
-

- $0.00 / 1KG
-
2020-05-12
- CAS:3416-26-0
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 500 MT
|
| | LIDOFLAZINE, 98.5% Basic information |
| Product Name: | LIDOFLAZINE, 98.5% | | Synonyms: | 6’-xylidide,4-(4,4-bis(p-fluorophenyl)butyl)-1-piperazineaceto-2;clinium;corflazine;klinium;lidoflazin;lidoflazina;lidoflazinum;mcn-jr7904 | | CAS: | 3416-26-0 | | MF: | C30H35F2N3O | | MW: | 491.62 | | EINECS: | 222-312-8 | | Product Categories: | | | Mol File: | 3416-26-0.mol | 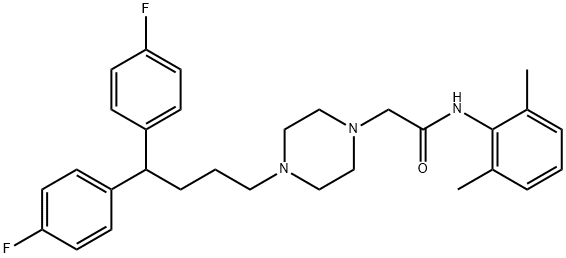 |
| | LIDOFLAZINE, 98.5% Chemical Properties |
| Melting point | 159-161° | | Boiling point | 632.6±55.0 °C(Predicted) | | density | 1.1322 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO: ≥10mg/mL | | pka | 14.62±0.70(Predicted) | | form | powder | | color | white | | InChIKey | ZBIAKUMOEKILTF-UHFFFAOYSA-N | | SMILES | Cc1cccc(C)c1NC(=O)CN2CCN(CCCC(c3ccc(F)cc3)c4ccc(F)cc4)CC2 | | CAS DataBase Reference | 3416-26-0 |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36 | | WGK Germany | 3 | | RTECS | TK7910000 | | Storage Class | 11 - Combustible Solids |
| | LIDOFLAZINE, 98.5% Usage And Synthesis |
| Chemical Properties | WHITE TO ALMOST WHITE POWDER | | Originator | Clinium,Janssen,W. Germany,1969 | | Uses | Coronary vasodilatator;Ca++ channel activator | | Uses | Lidoflazine is a L-type Ca2+ channel antagonist.Also, it is derived from 1,1''-(4-Chlorobutylidene)bis(4-fluorobenzene) (C364775), which is a derivative of Zerumbone with potential anti-tumor effects towards HeLa cancer cells. | | Definition | ChEBI: 2-[4-[4,4-bis(4-fluorophenyl)butyl]-1-piperazinyl]-N-(2,6-dimethylphenyl)acetamide is a diarylmethane. | | Manufacturing Process | A mixture of 6.6 parts 1-[4,4-di-(4-fluoro-phenyl)butyl]-piperazine, 4.33 parts
N-(2-chloro-acetyl)-2,6-dimethyl-aniline, 3.2 parts sodium carbonate, a few
crystals of potassium iodide in 200 parts 4-methyl-2-pentanone is stirred and
refluxed for 70 hours. After cooling there are added 70 parts water. The
organic layer is separated, dried over potassium carbonate, filtered and
evaporated. The oily residue is dissolved in 80 parts diisopropylether and the
solution is filtered hot. After cooling the filtrate at 0°C. the formed solid is
filtered off and recystallired from 80 parts ether, yielding 1-[4,4-di-(4-fluoro�phenyl)butyl]-4-[(2,6-dimethylanilino-carbonyl)-methyl]-piperazine; MP 159°C
to 161°C. | | Brand name | Angex (Janssen);
Clinium (Ortho-McNeil). | | Therapeutic Function | Coronary vasodilator | | Biological Activity | Lidoflazine is an antianginal calcium channel blocker th at carries a significant risk of QT interval prolongation and ventricular arrhythmia. It prolongs QT interval by blocking HERG channel. IC50 < 0.1 μM |
| | LIDOFLAZINE, 98.5% Preparation Products And Raw materials |
|