Sparsentan manufacturers
- Sparsentan
-

- $53.00 / 5mg
-
2026-02-01
- CAS:254740-64-2
- Min. Order:
- Purity: 98.75%
- Supply Ability: 10g
- Sparsentan
-

- $0.00 / 25kg
-
2025-12-01
- CAS:254740-64-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000kg
- Sparsentan
-

- $0.00 / 1g
-
2025-09-11
- CAS:254740-64-2
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
Related articles - Is sparsentan an FDA-approved drug?
- The US Food and Drug Administration has granted full approval to Travere Therapeutics’ sparsentan (Filspari) for slowing kidne....
- Jan 20,2025
|
| | Sparsentan Basic information |
| Product Name: | Sparsentan | | Synonyms: | 4'-((2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl)-N-(4,5-dimethylisoxazol-3-yl)-2'-(ethoxymethyl)-[1,1'-biphenyl]-2-sulfonamide;Sparsentan (RE-021);Sparsentan-d5 (RE-021-d5);RE 201;[1,1'-Biphenyl]-2-sulfonamide, 4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-;Sparsentan;4'-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-[1,1'-biphenyl]-2-sulfonamide;BMS-346567 | | CAS: | 254740-64-2 | | MF: | C32H40N4O5S | | MW: | 592.75 | | EINECS: | | | Product Categories: | API | | Mol File: | 254740-64-2.mol | 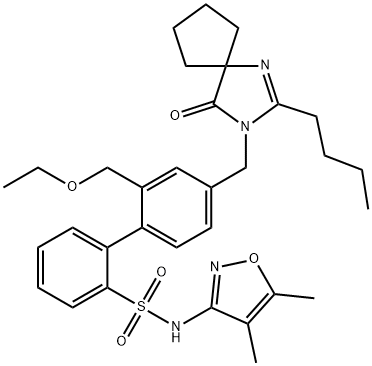 |
| | Sparsentan Chemical Properties |
| Melting point | 148 °C(Solv: isopropanol (67-63-0); water (7732-18-5)) | | Boiling point | 744.4±70.0 °C(Predicted) | | density | 1.28±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMSO: soluble | | pka | 7.06±0.50(Predicted) | | form | A crystalline solid | | color | White to off-white | | InChIKey | WRFHGDPIDHPWIQ-UHFFFAOYSA-N | | SMILES | C1(C2=CC=C(CN3C(=O)C4(CCCC4)N=C3CCCC)C=C2COCC)=CC=CC=C1S(NC1C(C)=C(C)ON=1)(=O)=O |
| WGK Germany | WGK 3 | | Storage Class | 11 - Combustible Solids |
| | Sparsentan Usage And Synthesis |
| Description | Sparsentan is an oral, dual endothelin angiotensin receptor antagonist that is being developed by Travere Therapeutics for the treatment of immunoglobulin A (IgA) nephropathy and focal segmental glomerulosclerosis (FSGS).[1] | | Uses | RE 201 is used an Endothelin A receptor antagonist used in the treatment of hypertension. | | Definition | Sparsentan (RE-021) is a selective dual-acting receptor antagonist with affinity for endothelin (A type) and angiotensin II receptors (Type 1). Sparsentan combines the active moieties of the selective AT1 receptor antagonist irbesartan and a selective ETA receptor antagonist. | | Biological Activity | Sparsentan (RE-021) is a highly potent dual angiotensin II and endothelin A receptor antagonist with Kis of 0.8 and 9.3 nM, respectively. | | Side effects |
Common side effects of Sparsentan include swelling of the hands, legs, ankles and feet; dizziness; and low red blood cells (anaemia).
Serious side effects of Sparsentan include hives; difficulty breathing; swelling of the face, lips, tongue, or throat; nausea; vomiting; stomach pain (upper right side); tiredness; loss of appetite; yellowing of the skin or eyes (jaundice); dark urine; fever; itching; severe birth defects low blood pressure; dizziness; lightheadedness; fainting; muscle cramps; dry skin; shortness of breath; trouble sleeping; frequent urination; little or no urination; abnormal blood test results (increased potassium); unusual weight gain and swelling of the ankles or legs.
| | in vitro | Sparsentan dose dependently antagonizes the angiotensin II-induced pressor response with an ED50 value of 0.8 μmol/kg iv and 3.6 μmol/kg po. Sparsentan also shows efficacious and long acting in the big ET-1-induced pressor model. Sparsentan causes a significant lowering of blood pressure at the lowest dose tested (10 μmol/kg/day) in spontaneously hypertensive rats. Sparsentan shows good oral bioavailability in rats, dogs, and monkeys, averaging 40%, 86%, and 21% F, respectively. At 100 μmol/kg/day, Sparsentan reduces the blood pressure from 170 to less than 100 mmHg during the course of the drug’s pharmacokinetic duration. Sparsentan at 100 μmol/kg/day essentially converts the spontaneously hypertensive rats into normotensive rats during the course of its pharmacokinetic duration. | | References | [1] Syed, Yahiya Y. “Sparsentan: First Approval.” Drugs 83 6 (2023): 563–568. |
| | Sparsentan Preparation Products And Raw materials |
|