1-Methyl-1H-indazol-4-ylamine manufacturers
|
| | 1-Methyl-1H-indazol-4-ylamine Basic information |
| Product Name: | 1-Methyl-1H-indazol-4-ylamine | | Synonyms: | 1-Methyl-1H-indazol-4-ylamine;1-Methyl-1H-indazole-4-amine;1-Methyl-1H-indazol-4-amine;1H-Indazol-4-aMine,1-Methyl-;4-AMino-1-Methylindazole;1-methylindazol-4-amine;1-Methyl-4-aMino-1h-indazole;1-Methyl-1H-indazol-4-amine, CAS 77894-69-0 | | CAS: | 77894-69-0 | | MF: | C8H9N3 | | MW: | 147.18 | | EINECS: | | | Product Categories: | pharmacetical | | Mol File: | 77894-69-0.mol | 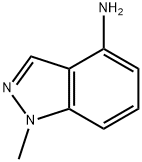 |
| | 1-Methyl-1H-indazol-4-ylamine Chemical Properties |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | form | solid | | color | Light yellow to brown |
| | 1-Methyl-1H-indazol-4-ylamine Usage And Synthesis |
| Synthesis | General procedure for the synthesis of 1-methyl-1H-indazol-4-amine from 1-methyl-4-nitro-1H-indazole: A catalytic amount of 10% palladium carbon was added to a solution of 1-methyl-4-nitro-1H-indazole (0.97 g, 5.5 mmol) in ethanol (50 ml). The resulting mixture was stirred under hydrogen atmosphere for 2 hours. Upon completion of the reaction, the catalyst was removed by filtration followed by evaporation of the solvent. The product was dried by azeotropy with toluene to give 1-methyl-1H-indazol-4-amine as a light brown solid (0.78 g, 96% yield). Mass spectrum (electrospray positive ion mode) m/z: 148 ([M+H]+). | | References | [1] Patent: WO2004/46133, 2004, A1. Location in patent: Page 28
[2] Patent: WO2005/28445, 2005, A2. Location in patent: Page/Page column 36
[3] Heterocycles, 2006, vol. 68, # 12, p. 2595 - 2605
[4] Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 14, p. 4740 - 4749
[5] Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 17, p. 6025 - 6035 |
| | 1-Methyl-1H-indazol-4-ylamine Preparation Products And Raw materials |
|