- Gefarnate
-

- $56.00 / 200mg
-
2026-02-01
- CAS:51-77-4
- Min. Order:
- Purity: 98.36%
- Supply Ability: 10g
- Gefarnate
-

- $15.00 / 1KG
-
2021-07-02
- CAS:51-77-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Gefarnate
-

- $7.00 / 1KG
-
2019-07-06
- CAS: 51-77-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1000KG
|
| | Gefarnate Basic information |
| Product Name: | Gefarnate | | Synonyms: | Gifarnate;Geranyl farnesylacetate;4,8,12-Tetradecatrienoic acid, 5,9,13-trimethyl-, (2E)-3,7-dimethyl-2,6-octadienyl ester, (4E,8E)-;GEFRNATE;[(2E)-3,7-Dimethylocta-2,6-dienyl] (4E,8E)-5,9,13-trimethyltetradeca-4,8,12-trienoate;(4E,8E)-5,9,13-Trimethyl-4,8,12-tetradecatrienoic acid (E)-3,7-dimethyl-2,6-octadienyl;Arsanate;Gefarnyl | | CAS: | 51-77-4 | | MF: | C27H44O2 | | MW: | 400.64 | | EINECS: | 200-121-0 | | Product Categories: | | | Mol File: | 51-77-4.mol | 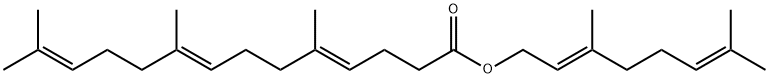 |
| | Gefarnate Chemical Properties |
| Melting point | 25°C | | Boiling point | bp0.05 165-168° | | density | 1.0044 (rough estimate) | | refractive index | nD20 1.4900 | | storage temp. | 2-8°C | | solubility | DMSO:31.25(Max Conc. mg/mL);78.0(Max Conc. mM) | | form | Oil | | color | Clear Colourless | | Stability: | Light Sensitive | | InChI | 1S/C27H44O2/c1-22(2)12-8-14-24(5)16-10-17-25(6)18-11-19-27(28)29-21-20-26(7)15-9-13-23(3)4/h12-13,16,18,20H,8-11,14-15,17,19,21H2,1-7H3/b24-16+,25-18+,26-20+ | | InChIKey | ZPACYDRSPFRDHO-ROBAGEODSA-N | | SMILES | CC(C)=CCC/C(C)=C/COC(CC/C=C(CC/C=C(CCC=C(C)C)\C)\C)=O | | EPA Substance Registry System | 4,8,12-Tetradecatrienoic acid, 5,9,13-trimethyl-, (2E)-3,7-dimethyl-2,6-octadienyl ester, (4E,8E)- (51-77-4) |
| WGK Germany | WGK 3 | | TSCA | TSCA listed | | Storage Class | 10 - Combustible liquids |
| | Gefarnate Usage And Synthesis |
| Uses | Gefarnate is a synthetic isoprenoid that is used to treat chronic gastric and duodenic ulcers. Gerfarnate is also used for gastric ulcer prophylaxis. | | Definition | ChEBI: Gefarnate is an organic molecular entity. | | Biological Activity | Gefarnate is an anti-ulcer drug th at stimulates gastric mucin secretion. Also gefarnate increase secretion of mucin-like glycoprotein in rabbit conjunctival tissue. It appears to be effective in animal dry-eye models. It is approved in Japan, where it is used to tre at gastritis and gastric ulcer. Gefarnate is structurally similar to anti-ulcer drug teprenone (geranyl farnesylacetate), which induces expression of HSP70, HSPB8, and HSPB1. | | Synthesis | The general procedure for the synthesis of (4E,8E)-(E)-3,7-dimethylocta-2,6-dien-1-yl 5,9,13-trimethyltetradeca-4,8,12-trienoate from geraniol and (4E,8E)-5,9,13-trimethyltetradeca-4,8,12-trienoate was performed as follows: 10.3 g farnesyl acetic acid, 15.0 g g geraniol, 25.0 mL xylene and 0.43 g hydroquinone were mixed and refluxed for about 10 hours. The progress of the reaction was monitored during the reaction using thin layer chromatography (TLC with a spreader ratio of petroleum ether/ethyl acetate = 25:1) and the complete reaction of farnesylacetic acid was confirmed by TLC (spreader ratio of petroleum ether/ethyl acetate = 5:1). Upon completion of the reaction, an orange-yellow transparent mixed solution was obtained, which was slightly cooled and xylene was removed by distillation under reduced pressure. The residue was extracted 2-3 times with hexane and 1% (w/w) sodium hydroxide solution, the organic phase was separated, dried and some of the solvent was removed by rotary evaporation. The residue was dried over anhydrous calcium chloride and the mixture was stirred overnight at room temperature and the filtrate was collected by filtration. The filtrate was concentrated to give the crude product of gefarnate ester. The crude product was purified by reduced pressure distillation and the 186-200 °C fraction was collected to give the pure gefarnate ester product. | | References | [1] Patent: CN103012140, 2016, B. Location in patent: Paragraph 0071; 0074; 0093 |
| | Gefarnate Preparation Products And Raw materials |
|