- Lonicerin
-

- $52.00 / 1mg
-
2026-02-02
- CAS:25694-72-8
- Min. Order:
- Purity: 99.91%
- Supply Ability: 10g
- Lonicerin
-
- $0.00 / 10mg
-
2023-02-24
- CAS:25694-72-8
- Min. Order: 10mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
- Lonicerin
-

- $7.00 / 1KG
-
2020-02-17
- CAS: 25694-72-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| | Lonicerin Basic information |
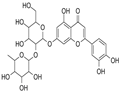
| Product Name: | Lonicerin | | Synonyms: | 7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-oxan-2-yl]oxy-oxan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5-hydroxy-chromen-4-one;Scolymoside;veronicastroside;LUTEOLIN-7-RUTINOSIDE;LONICERIN;Luteolin 7-beta-neohesperidoside;Luteolin-7-neohesperidoside;Lonicerin Luteolin 7-neohesperidoside | | CAS: | 25694-72-8 | | MF: | C27H30O15 | | MW: | 594.52 | | EINECS: | | | Product Categories: | | | Mol File: | 25694-72-8.mol |  |
| | Lonicerin Chemical Properties |
| Boiling point | 957.8±65.0 °C(Predicted) | | density | 1.76±0.1 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | solubility | DMF: 50 mg/ml; DMSO: 50 mg/ml | | pka | 6.08±0.40(Predicted) | | form | A crystalline solid | | color | Light yellow to yellow | | InChIKey | SHPPXMGVUDNKLV-KMFFXDMSSA-N | | SMILES | O1[C@H]([C@@H]([C@@H]([C@H]([C@@H]1C)O)O)O)O[C@H]2[C@@H](O[C@@H]([C@H]([C@@H]2O)O)CO)Oc3cc4[o]c(c[c](c4c(c3)O)=O)c5cc(c(cc5)O)O |
| WGK Germany | WGK 3 | | Storage Class | 11 - Combustible Solids |
| | Lonicerin Usage And Synthesis |
| Uses | Lonicerin is an anti-algE (alginate secretion protein) flavonoid with inhibitory activity for P. aeruginosa. Lonicerin prevents inflammation and apoptosis in LPS-induced acute lung injury[1][2]. | | Definition | ChEBI: A disaccharide derivative that is luteolin substituted by a 2-O-(alpha-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosidic linkage. | | References | [1] Xu Z, et al. Lonicerin, an anti-algE flavonoid against Pseudomonas aeruginosa virulence screened from Shuanghuanglian formula by molecule docking based strategy. J Ethnopharmacol. 2019 Jul 15;239:111909. DOI:10.1016/j.jep.2019.111909
[2] Lee JH, et al. Antiarthritic effect of lonicerin on Candida albicans arthritis in mice. Arch Pharm Res. 2011 May;34(5):853-9.
[3] Xiao Ding, et al. Evaluation of Anti-MRSA and Xanthine Oxidase Inhibition Activities of Phenolic Constituents from Plumula nelumbinis. Journal of Chemistry. 24 December 2015. |
| | Lonicerin Preparation Products And Raw materials |
|