|
|
| | ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydropyridine-3-carboxylate Basic information |
| Product Name: | ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydropyridine-3-carboxylate | | Synonyms: | ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydropyridine-3-carboxylate;1,4,5,6-Tetrahydro-6-oxo-2-(trifluoromethyl)-3-pyridinecarboxylic acid ethyl ester;6-Hydroxy-2-trifluoromethyl-4,5-dihydropyridine-3-carboxylic acid ethyl ester;-1,4,5,6-tetrahydropyridine-3-carboxylate;Ethyl 6-oxo-2-(trifluoromethyl);Ethyl 2-oxo-6-(trifluoromethyl)-3,4-dihydro-1h-pyridine-5-carboxylate;3-Pyridinecarboxylicacid, 1,4,5,6-tetrahydro-6-oxo-2-(trifluoromethyl)-, ethyl ester;2-oxo-6-(trifluoromethyl)-3,4-dihydro-1H-pyridine-5-carboxylic acid ethyl ester | | CAS: | 194673-12-6 | | MF: | C9H10F3NO3 | | MW: | 237.18 | | EINECS: | | | Product Categories: | Heterocycles | | Mol File: | 194673-12-6.mol | 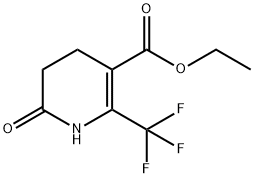 |
| | ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydropyridine-3-carboxylate Chemical Properties |
| Melting point | 128~130℃ | | Boiling point | 294℃ | | density | 1.351 | | Fp | 132℃ | | storage temp. | Sealed in dry,Room Temperature | | pka | 11.80±0.60(Predicted) | | Appearance | Off-white to light yellow Solid |
| | ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydropyridine-3-carboxylate Usage And Synthesis |
| Synthesis | GENERAL STEPS: A mixture of ethyl 4,4,4-trifluoroacetoacetate (14.7 mL, 0.1 mol, 1.6 eq.), acrylamide (4.5 g, 0.063 mol, 1.0 eq.), and p-toluene sulfonate (0.156 g, 0.82 mmol, 0.013 eq.) in toluene (60 mL) was refluxed in a Dean-Stark device for for 38 hours to azeotropically remove the resulting water. Upon completion of the reaction, most of the toluene was removed by slow distillation at atmospheric pressure and the reaction mixture was concentrated to a smaller volume. Toluene (60 mL) was added again to the concentrated mixture and the distillation and concentration process was repeated as described above. This operation was performed three times. Finally, the reaction mixture was further concentrated under vacuum and the resulting solid residue was purified by fast column chromatography (silica gel as stationary phase, eluent gradient: from hexane/ethyl acetate 9:1 to hexane/ethyl acetate 8:2). Purification afforded the target compound ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydro-3-pyridinecarboxylate as a brown solid (3.8 g, 25% yield). The product was analyzed by LC-MS (ESI+) and showed molecular ion peaks MH+: 238, 210, 190. | | References | [1] Patent: WO2005/74939, 2005, A1. Location in patent: Page/Page column 40; 44
[2] Patent: WO2004/29026, 2004, A1. Location in patent: Page 33-34
[3] Patent: WO2004/29027, 2004, A1. Location in patent: Page 30 |
| | ethyl 6-oxo-2-(trifluoromethyl)-1,4,5,6-tetrahydropyridine-3-carboxylate Preparation Products And Raw materials |
|