|
|
| | HUMULONE Basic information |
| Product Name: | HUMULONE | | Synonyms: | (6S)-3,5,6-trihydroxy-2-(3-methylbutanoyl)-4,6-bis(3-methylbut-2-enyl)cyclohexa-2,4-dien-1-one;(6R)-3,5,6-trihydroxy-2-isovaleryl-4,6-bis(3-methylbut-2-enyl)cyclohexa-2,4-dienone;α-bitter acid;Humulone, mixture of homologues;α-Bitter acid, α-Lupulic acid;(6R)-2-(3-Methylbutyryl)-3,5,6-trihydroxy-4,6-bis(3-methyl-2-butenyl)-2,4-cyclohexadiene-1-one;(6R)-4,6-Diprenyl-3,5,6-trihydroxy-2-(1-oxo-3-methylbutyl)-2,4-cyclohexadiene-1-one;[R,(-)]-3,5,6-Trihydroxy-4,6-bis(3-methyl-2-butenyl)-2-(3-methyl-1-oxobutyl)-2,4-cyclohexadien-1-one | | CAS: | 26472-41-3 | | MF: | C21H30O5 | | MW: | 362.46 | | EINECS: | 247-725-0 | | Product Categories: | Miscellaneous Natural Products | | Mol File: | 26472-41-3.mol | 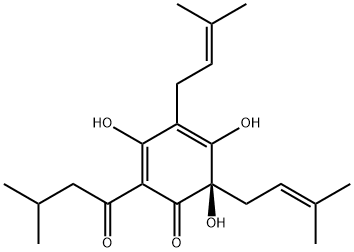 |
| | HUMULONE Chemical Properties |
| Melting point | 65-66.5℃ | | alpha | D20 -212° (1.0 g in 15.5 g 96% alc) | | Boiling point | 414.06°C (rough estimate) | | density | 1.157±0.06 g/cm3 (20 ºC 760 Torr) | | refractive index | 1.6120 (estimate) | | storage temp. | −20°C | | solubility | DMSO : 100 mg/mL (275.89 mM; Need ultrasonic) | | pka | 4.50±1.00(Predicted) | | form | Powder | | color | Light yellow to yellow |
| | HUMULONE Usage And Synthesis |
| Uses | (R)-Humulone (CAS# 26471-41-3) can be used as a chemosensory receptor ligand. | | Definition | ChEBI: Humulone is an optically active cyclic ketone consisting of 3,5,6-trihydroxycyclohexa-2,4-dien-1-one bearing two 3-methylbut-2-en-1-yl substituents at positions 4 and 6 as well as a 3-methylbutanoyl group at the 2-position. It has a role as an antibacterial drug, an antioxidant, a cyclooxygenase 2 inhibitor and a metabolite. It is a diketone, a triol, a cyclic ketone, an aromatic ketone and a tertiary alpha-hydroxy ketone. | | Synthesis | The 700g crushed hops are loaded into the extractor, and suitable extraction pressure, extraction temperature, CO2 flow rate and extraction time are selected. CO2 enters into the extractor through cooling and pressurization, and in the heated and pressurized state, the active ingredient in the hops is dissolved in the CO2 in the liquid or supercritical state, and passes through the separator with the CO2 fluid. By lowering the pressure and increasing the temperature, the active ingredients and CO2 are transformed into gaseous state and separated from the separator, while the extract remains in the separator, and the separated CO2 gas is condensed and recycled. After the extraction time is reached, the operation is stopped and the sample is taken from the sampling valve at the bottom of the separator. When the system pressure is restored to atmospheric pressure, the hops extract can be unloaded and prepared for the next operation. Collect the hops extract from the extractor for subsequent experiments.
Weigh 100 mg of hops supercritical CO2 resin extract in a 10 mL volumetric flask, dissolve in methanol and make up to the scale. The mobile phase: methanol (A): 0.1% phosphoric acid (B) = 90:10; flow rate: 0.8 mL/min; injection volume: 10 ??L; column temperature: 25 ??; detection time: 20 min; detection wavelength: 315 nm. The extract was separated and purified. | | in vivo | Humulone (10 or 20 mg/kg; IP; single dose) shortens sleep onset and increases the duration of sleep induced by pentobarbital and decreases the spontaneous locomotion in open field at 20 mg/kg[2].
Humulone (10 μmol; applied topically to the dorsal shaved area) pre-treatment significantly inhibited TPA (10 nmol)-induced COX-2 expression in Female ICR mice (6-7 weeks of age) skin[3].
Humulone (1, 10 μmol; applied topical; pre-treatment 30 min) suppresses TPA-induced NF-κB DNA binding. Humulone attenuates TPA-stimulated nuclear translocation of p65 and p50 subunit proteins of NF-κB[3].
| Animal Model: | Male BALB/cAnNRj mice (9-11 weeks of age)[2] | | Dosage: | 10 or 20 mg/kg | | Administration: | IP; pre-treatment before sodium pentobarbital (35 mg/kg; i.p.) and ethanol (3.5 g/kg) | | Result: | Significantly decreased the latency and prolonged the duration of sleep induced by pentobarbital at 20 mg/kg dose. These effects were not observed at a lower dose of 10 mg/kg.
Showed no effect on the onset of sleep induced by ethanol, but significantly increased sleep duration dose-dependently.
|
| | target | IL Receptor | AP-1 | COX | p65 | NF-kB | IkB | VEGFR | Antifection | IKK | | IC 50 | COX-2 | | Purification Methods | Crystallise humulon from Et2O. It dissolves slightly in hot H2O but precipitates on cooling. It has max () 237 (13,760) and 282 (8,330) in EtOH. [Wollmer Chem Ber 49 780 1916, Carson J Am Chem Soc 73 4652 1951, Beilstein 8 II 537, 8 III 4034, 8 IV 3410.] |
| | HUMULONE Preparation Products And Raw materials |
|