|
|
| | Boc-DL-3-Aminoisobutyric acid Basic information | | Uses |
| Product Name: | Boc-DL-3-Aminoisobutyric acid | | Synonyms: | Boc-DL-3-Aminoisobutyric acid;3-(tert-butoxycarbonylamino)-2-methylpropanoic acid;REF DUPL: Boc-DL-3-Aminoisobutyric acid;Boc-DL-3-AMinoisobutyric acid Boc-DL-3-AMinoisobutyric acid;Propanoic acid, 3-[[(1,1-diMethylethoxy)carbonyl]aMino]-2-Methyl-, (±)-;3-(BOC-AMINO)-2-METHYLPROPANOIC ACID;3-(tert-butoxycarbonyl)-2-methylpropanoic acid;2-methyl-3-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid | | CAS: | 16948-10-0 | | MF: | C9H17NO4 | | MW: | 203.24 | | EINECS: | | | Product Categories: | | | Mol File: | 16948-10-0.mol | 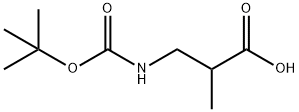 |
| | Boc-DL-3-Aminoisobutyric acid Chemical Properties |
| Melting point | 89-90℃ (ethyl ether pentane ) | | Boiling point | 339.5±25.0 °C(Predicted) | | density | 1.101±0.06 g/cm3 (20 ºC 760 Torr) | | storage temp. | 2-8°C | | pka | 4.53±0.10(Predicted) | | form | crystalline powder | | color | White | | Optical Rotation | Consistent with structure |
| HazardClass | IRRITANT | | HS Code | 2922498590 |
| | Boc-DL-3-Aminoisobutyric acid Usage And Synthesis |
| Uses | BOC-DL-3-aminoisobutyric acid is a product in which the amino group of 3-aminoisobutyric acid is protected by a tert-butoxycarbonyl (BOC) group, and it can be used as an intermediate in the synthesis of pharmaceuticals and chemicals. | | Synthesis | GENERAL STEPS: DL-3-aminoisobutyric acid (CAS No. [10569-72-9], 5 g, 47.52 mmol) was dissolved in 2N NaOH solution (24.7 mL), and a solution of tetrahydrofuran (THF, 75 mL) of di-tert-butyl dicarbonate (Boc2O, 11.73 g, 53.22 mmol) was added slowly dropwise with the cooling of a cold water bath, controlling the The reaction temperature was below 30 °C. The reaction mixture was stirred at room temperature for 18 hours and then concentrated under reduced pressure. The concentrate was diluted with water (75 mL) and washed with methyl tert-butyl ether (MTBE, 3 x 150 mL). The aqueous phase was acidified to pH 3 with 100 g/L citric acid solution (150 mL) and subsequently extracted with ethyl acetate (EtOAc, 3 × 150 mL). The organic phases were combined, washed with water (3×45 mL), dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure to afford 9.4 g of the target product 3-((tert-butoxycarbonyl)amino)-2-methylpropanoic acid (Compound 11) as a colorless oil in 97% yield. Nuclear magnetic resonance hydrogen spectrum (1H NMR, 400 MHz, DMSO-d6, δ, ppm): 1.01 (d, J = 7.1 Hz, 3H); 1.38 (s, 9H); 2.48 (m, 1H); 2.91 (dt, J = 6.1, 6.5 and 13.5 Hz, 1H); 3.15 (ddd, J = 6.1, 7.4 and 13.5 Hz , 1H); 6.80 (t, J = 6.1 Hz, 1H); 12.15 (broad s, 1H). | | References | [1] Journal of Organic Chemistry, 2001, vol. 66, # 20, p. 6541 - 6544
[2] Patent: WO2017/76998, 2017, A1. Location in patent: Page/Page column 163
[3] Patent: EP2380890, 2011, A1. Location in patent: Page/Page column 19
[4] Patent: CN108341752, 2018, A. Location in patent: Paragraph 0482; 0483; 0484; 0485
[5] European Journal of Organic Chemistry, 2012, # 29, p. 5774 - 5788,15 |
| | Boc-DL-3-Aminoisobutyric acid Preparation Products And Raw materials |
|