|
|
| | 3-AMINO-4-BROMO-1H-INDAZOLE Basic information |
| Product Name: | 3-AMINO-4-BROMO-1H-INDAZOLE | | Synonyms: | 3-AMINO-4-BROMO-1H-INDAZOLE;1H-Indazol-3-aMine, 4-broMo-;4-Bromo-1H-indazol-3-amine;4-BroMo-1H-indazol-3-ylaMine;4-bromo-2H-indazol-3-amine;4-Bromo-1H-indazol-4-amine | | CAS: | 914311-50-5 | | MF: | C7H6BrN3 | | MW: | 212.05 | | EINECS: | | | Product Categories: | | | Mol File: | 914311-50-5.mol | 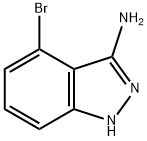 |
| | 3-AMINO-4-BROMO-1H-INDAZOLE Chemical Properties |
| Melting point | 142-144℃ | | Boiling point | 431.3±25.0 °C(Predicted) | | density | 1.867±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | Slightly soluble. | | pka | 13.68±0.40(Predicted) | | form | Crystalline Powder | | color | Off-white to brown to gray |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN2811 | | HazardClass | IRRITANT | | HS Code | 29339980 |
| | 3-AMINO-4-BROMO-1H-INDAZOLE Usage And Synthesis |
| Uses | Microwave heating of 2-halobenzaldehydes or 2-haloacetophenones with phenylhydrazines at 160°C for 10 min quantitatively yielded the arylhydrazones, which were further cyclized to give 1-aryl-1H-indazoles via CuI/diamine-catalyzed N-arylation under microwave heating (160°C, 10 min). Good to excellent yields were observed for 2-iodo, 2-bromo, and 2-chloro benzaldehydes or acetophenones. | | Synthesis | A solution of 2-fluoro-6-bromobenzonitrile (22.0 mmol) and 98% hydrazine hydrate (90.0 mmol) in n-butanol (15 ml) was stirred at reflux for 7 hours. The mixture was cooled to room temperature. The resulting mixture was concentrated under reduced pressure to a volume of 5 ml. water was added to the resulting mixture. The solid product was precipitated. The precipitate was filtered. Wash the precipitate with water. Dry the precipitate. The precipitate was purified by crystallization from methanol to give 4-bromo-1H-indazol-3-amine. |
| | 3-AMINO-4-BROMO-1H-INDAZOLE Preparation Products And Raw materials |
|