| Company Name: |
ATK CHEMICAL COMPANY LIMITED |
| Tel: |
+undefined-21-51877795 |
| Email: |
ivan@atkchemical.com |
| Products Intro: |
Product Name:9H-Imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, 11,12,13,13a-tetrahydro-7-methoxy-9-oxo-, ethyl ester, (13aS)-
CAS:130477-52-0
Purity:98% Package:10MG;50MG;100MG,1G,5G,10G
|
|
|
|
|
|
| | L-655,708 Basic information |
| Product Name: | L-655,708 | | Synonyms: | ETHYL (S)-11,12,13,13A-TETRAHYDRO-7-METHOXY-9-OXO-9H-IMIDAZO[1,5-A]PYRROLO[2,1-C][1,4]BENZODIAZEPINE-1-CARBOXYLATE;L-655,708;11,12,13,13A-TETRAHYDRO-7-METHOXY-9-OXO-9H-IMIDAZO[1,5-A]PYRROLO[2,1-C][1,4]BENZODIAZEPINE-1-CARBOXYLIC ACID, ETHYL ESTER;11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1.5-a]pyrrolo[2.1-c][1.4]benzodiazepine-1-carboxylicacidethylester;Ethyl (S)-11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate;MSD;9H-IMidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, 11,12,13,13a-tetrahydro-7-Methoxy-9-oxo-, ethyl ester, (13aS)- (011-14426_500Mg);9H-IMidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, 11,12,13,13a-tetrahydro-7-Methoxy-9-oxo-, ethyl ester, (13aS)- | | CAS: | 130477-52-0 | | MF: | C18H19N3O4 | | MW: | 341.36 | | EINECS: | | | Product Categories: | GABA/Glycine receptor | | Mol File: | 130477-52-0.mol | 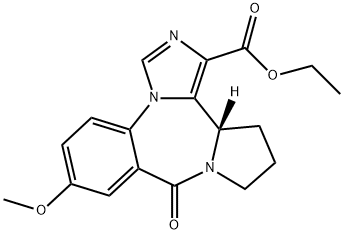 |
| | L-655,708 Chemical Properties |
| Melting point | 175-176 °C | | Boiling point | 584.4±50.0 °C(Predicted) | | density | 1.42±0.1 g/cm3(Predicted) | | storage temp. | Desiccate at +4°C | | solubility | DMSO: 6 mg/mL | | form | powder | | pka | 1.49±0.20(Predicted) | | color | White to yellow | | InChI | 1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | | InChIKey | YKYOQIXTECBVBB-AWEZNQCLSA-N | | SMILES | CCOC(=O)c1ncn2-c3ccc(OC)cc3C(=O)N4CCCC4c12 |
| WGK Germany | 3 | | Storage Class | 11 - Combustible Solids |
| | L-655,708 Usage And Synthesis |
| Uses | L-655,708 has been used as an α5 GABAA receptor inverse agonist to inhibit the discriminative stimulus of propofol in a dose-dependent manner. | | Biological Activity | Potent, selective inverse agonist for the benzodiazepine site of GABA A receptors containing the α 5 subunit (K i = 0.45 nM). Displays 50-100-fold selectivity over GABA A receptors containing α 1, α 2, α 3 or α 6 subunits in combination with β 3 and γ 2. Enhances LTP in? a mouse hippocampal slice model and increases spatial learning, without displaying proconvulsant activity. | | Biochem/physiol Actions | L-655,708 is an inverse agonist of the α5 γ-Aminobutyric acid type A (GABAA) receptor. It has an ability to increase cognition in rats. | | in vivo | L-655708 at 0.7 mg/kg, administered intraperitoneally, would result in 60-70% occupancy of α5 GABAA receptors with limited binding to α1, α2, and α3 subunit-containing GABAA receptors and no significant off-target behavioral effects, such as sedation and motor impairment. | | storage | Desiccate at +4°C |
| | L-655,708 Preparation Products And Raw materials |
|