Belzutifan manufacturers
- Belzutifan
-

- $64.00 / 1mg
-
2026-01-13
- CAS:1672668-24-4
- Min. Order:
- Purity: 99.68%
- Supply Ability: 10g
- Belzutifan
-

- $780.00 / 1g
-
2024-05-28
- CAS:1672668-24-4
- Min. Order: 1g
- Purity: 98
- Supply Ability: 500 Kg
Related articles - How is Belzutifan synthesised?
- Belzutifan was prepared by a synthetic route starting from 3,4-dihydrocoumarin, followed sequentially by the synthesis of Inda....
- Jan 29,2024
|
| | Belzutifan Basic information |
| Product Name: | Belzutifan | | Synonyms: | Benzonitrile, 3-[[(1S,2S,3R)-2,3-difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1H-inden-4-yl]oxy]-5-fluoro-;PT2977;MK-6482;PT2977 (MK-6482);Belzutifan (MK-6482);Belzutifan;Belzutifan (PT2977, MK-6482);HY-125840 Belzutifan PT2977 | | CAS: | 1672668-24-4 | | MF: | C17H12F3NO4S | | MW: | 383.34 | | EINECS: | | | Product Categories: | | | Mol File: | 1672668-24-4.mol | 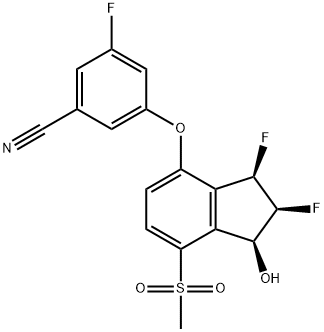 |
| | Belzutifan Chemical Properties |
| Boiling point | 505.8±50.0 °C(Predicted) | | density | 1.56±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C, stored under nitrogen | | solubility | DMSO: 50 mg/mL (130.43 mM) | | form | Solid | | pka | 11.66±0.60(Predicted) | | color | White to light yellow | | InChI | InChI=1S/C17H12F3NO4S/c1-26(23,24)12-3-2-11(13-14(12)17(22)16(20)15(13)19)25-10-5-8(7-21)4-9(18)6-10/h2-6,15-17,22H,1H3/t15-,16-,17+/m1/s1 | | InChIKey | LOMMPXLFBTZENJ-ZACQAIPSSA-N | | SMILES | C(#N)C1=CC(F)=CC(OC2=CC=C(S(C)(=O)=O)C3=C2[C@@H](F)[C@@H](F)[C@H]3O)=C1 |
| | Belzutifan Usage And Synthesis |
| Description | Belzutifan is an orally active, small molecule inhibitor of hypoxia inducible factor (HIF)-2alpha (HIF-2a), with potential antineoplastic activity. The HIF-2α protein was a key player in the growth of certain cancers. Initially considered to be undruggable, a binding pocket was eventually discovered in the HIF-2α molecule which allowed for compounds to bind and inhibit these proteins. | | Chemical Properties | PT2977 has high stability and is able to keep its structure and properties stable in a variety of environments; it also has good solubility and reactivity and is able to undergo a variety of chemical reactions with other compounds; in addition, PT2977 has good optical and electrical properties. | | Uses | Belzutifan is a prescription medicine used to treat adults with von Hippel-Lindau (VHL) disease who need treatment for a type of kidney cancer called renal cell carcinoma (RCC), tumors in the brain and spinal cord called central nervous system hemangioblastomas, or a type of pancreatic cancer called pancreatic neuroendocrine tumors, that do not need surgery right away. | | Indications | Belzutifan is indicated for the treatment of adult patients with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET), who do not require immediate surgery. | | Mechanism of action | Belzutifan inhibits the complexation of HIF-2α with another transcription factor, HIF-1β, a necessary step in its activation - by preventing the formation of this complex, belzutifan can slow or stop the growth of VHL-associated tumors. Belzutifan received FDA approval for the treatment of select VHL-associated cancers on August 13, 2021. | | Pharmacokinetics | Upon oral administration, belzutifan binds to and blocks the function of HIF-2alpha, thereby preventing HIF-2alpha heterodimerization and its subsequent binding to DNA. This results in decreased transcription and expression of HIF-2alpha downstream target genes, many of which regulate hypoxic signaling. This inhibits cell growth and survival of HIF-2alpha-expressing tumor cells. HIF-2alpha, the alpha subunit for the heterodimeric transcription factor HIF-2, is overexpressed in many cancers and promotes tumorigenesis. | | Side effects | Side effects ofBelzutifan that was reported:
allergic reactions(skin rash,itchingorhives; swelling of the face, lips, or tongue)
blurred vision OR changes in vision
high blood sugar (increased hunger, thirst or urination; unusually weak or tired, blurry vision)
infection (fever, chills,cough,sore throat,pain;or trouble passing urine)
low calcium levels(fast heartbeat; muscle cramps or;pain;;pain, tingling, or numbness in the hands or feet; seizures)
low red blood cell counts (feeling faint; lightheaded, falls; unusually weak or tired)
trouble breathing
trouble passing urine or change in the amount of urine | | Synthesis | Belzutifan’s route started with 3,4-dihydrocoumarin (33.1) and proceeded in three steps:
Step 1: Synthesis of the indanone core 33.8. 3,4-Dihydrocoumarin (33.1) could be regioselectively brominated with NBS under Lewis acid conditions to generate intermediate 33.2, paving the way for the subsequent tandem lactone ring opening/SNAr reaction (Figure 1). The desired ring-opening product was successfully generated by treating 33.2 with K2CO3 in NMP and H2O, which spontaneously participated in the SNAr reaction in the presence of 3,5-difluorobenzonitrile (33.3) and 18-crown-6 to generate intermediate 33.5. After isopropanoate (i-PrOAc) extraction, the crude product was reacted with oxalyl chloride in MTBE/DMF to generate the corresponding acyl chloride 33.6, which underwent an intramolecular Friedel–Crafts reaction in the presence of AlCl3. This series of reactions started with the bromination of intermediate 33.2 to generate indanone 33.7. The introduction of methyl sulfone was achieved using sodium methanesulfonate under copper-mediated conditions to generate 33.8 in 78% yield.

Step 2: Synthesis of indanone ketal 33.13. Starting from indanone 33.8, the keto group was protected to its corresponding ketal intermediate 33.9 before developing a continuous-flow light-mediated benzylic bromination step (Figure 2). Thus, intermediate 33.11 could be free-radical brominated with 1,3-dibromo-5,5-dimethylhydantoin (33.10) in acetonitrile in the presence of citric acid and visible light to generate a crude reaction mixture that was carefully quenched with 2,6-butane and 1,3-dimethoxybenzene. The brominated product 33.11, which showed limited stability when isolated, was directly oxidized in a continuous flow process to generate the ketone 33.13 via a modified Kornblum oxidation with 2-methylpyridine N-oxide (33.12).

Step 3: The presence of three consecutive chiral centers on the indene ring was ensured. First, the introduction of the chiral alcohol 33.14 was accomplished by asymmetric reduction under transfer hydrogenation conditions using (R,R)-Ru-diphenylethylenediamine ((R,R)-Ru-DPEN) and formic acid/triethylamine in acetonitrile to afford the (R)-hydroxyketone with high selectivity (Figure 3). The reduction step was immediately quenched with aqueous hydrochloric acid to promote the cleavage of the acetal group, resulting in the crystallization of intermediate 33.14 from water in 91% overall yield and greater than 99% enantiomeric excess. Next, intermediate 33.14 was α-fluorinated by reaction with Selectfluor and catalytic methanesulfonic acid to generate a mixture of fluorinated stereoisomers (33.15), which immediately underwent Noyori asymmetric transfer hydrogenation after quenching the excess fluorinating agent with triethylamine. Under these hydrogenation conditions (RuCl(R,R)-TsDPEN/triethylamine/formic acid/methanesulfonic acid), the reduction of the ketone occurred with high selectivity while promoting the dynamic kinetic resolution (DKR) of the adjacent fluorinated substituents in a one-pot reaction. Although acid-mediated acetal byproducts were observed in this reaction sequence, the desired stereoisomer 33.16 was isolated from 33.14 in 80% yield (99:1 enantiomeric ratio) by quenching the reaction with water. Finally, the final stereocenter was established by deoxyfluorination of intermediate 33.16 using perfluoro-1-butanesulfonyl fluoride (PBSF, 33.17). In this reaction, selective deprotonation of the desired alcohol using DBU was crucial. This reaction completely reversed the stereochemistry and afforded Belzutifan (33) in 73% yield and 99.8% purity by recrystallization from water.
 |
| | Belzutifan Preparation Products And Raw materials |
|