- DO-(CH2COOt-Bu)3
-

- $0.00 / 25g
-
2025-06-07
- CAS:122555-91-3
- Min. Order: 25g
- Purity: >95.00%
- Supply Ability: 25g
|
| | TRI-T-BUTYL 1 4 7 10-TETRAAZACYCLODODECA Basic information |
| Product Name: | TRI-T-BUTYL 1 4 7 10-TETRAAZACYCLODODECA | | Synonyms: | TRI-T-BUTYL 1,4,7,10-TETRAAZACYCLODODECANE-1,4,7-TRIACETATE;1,4,7-Tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane;1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic Acid Tri-tert-butyl Ester;1,4,7,10-Tetraazacyclododecane-1,4,7-tris(t-butyl acetate)(DO3A-t-Butyl ester);DO3A-t-Bu-ester(M-130);1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid, tris(1,1-diMethylethyl) ester;1,4,7,10-TETRAAZACYCLODODECANE-1,4,7-TRIACETATE;TRI-T-BUTYL 1 4 7 10-TETRAAZACYCLODODECA | | CAS: | 122555-91-3 | | MF: | C26H50N4O6 | | MW: | 514.7 | | EINECS: | 635-018-3 | | Product Categories: | | | Mol File: | 122555-91-3.mol | 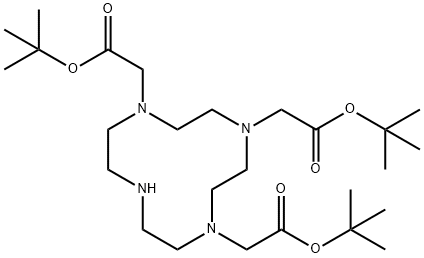 |
| | TRI-T-BUTYL 1 4 7 10-TETRAAZACYCLODODECA Chemical Properties |
| Melting point | 181-183℃ | | Boiling point | 561.6±50.0 °C(Predicted) | | density | 1.022 | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | form | Low-Melting Solid | | pka | 9.57±0.20(Predicted) | | color | White to Off-White | | Sensitive | Hygroscopic | | InChI | InChI=1S/C26H50N4O6/c1-24(2,3)34-21(31)18-28-12-10-27-11-13-29(19-22(32)35-25(4,5)6)15-17-30(16-14-28)20-23(33)36-26(7,8)9/h27H,10-20H2,1-9H3 | | InChIKey | NMHVTLJFPDOJOD-UHFFFAOYSA-N | | SMILES | N1(CC(OC(C)(C)C)=O)CCNCCN(CC(OC(C)(C)C)=O)CCN(CC(OC(C)(C)C)=O)CC1 |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26 | | WGK Germany | 3 | | HS Code | 29339900 |
| | TRI-T-BUTYL 1 4 7 10-TETRAAZACYCLODODECA Usage And Synthesis |
| Uses | Tri-tert-butyl 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetate with amide ligands bearing positively charged terbium complexes are used for sensitive detection of NADPH-dependent enzyme activities. | | Synthesis | Under nitrogen protection, 1,4,7,10-tetraazacyclododecane (1 g, 5.81 mmol) was dissolved in anhydrous acetonitrile (20 ml) and cooled to 0 °C. Sodium bicarbonate (1.464 g, 17.44 mmol) was added to the reaction system under stirring conditions. Subsequently, anhydrous acetonitrile solution (10 ml) of tert-butyl bromoacetate (3.4 g, 17.44 mmol) was slowly added through a dropping funnel over a period of 1 hour. The reaction mixture was continued to be stirred at 0 °C for 3 h, followed by raising to room temperature and stirring for 45 h. The progress of the reaction was monitored by thin layer chromatography (TLC) with the unfolding agent being dichloromethane (DCM): methanol (9:1), and the target product had an Rf value of 0.67. Upon completion of the reaction, the reaction mixture was filtered and the filtrate was concentrated to dryness under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent being dichloromethane solution containing 2% methanol to afford tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7-triacetate (Compound 2) as a white powder in 83.5% yield. | | References | [1] Tetrahedron Letters, 2013, vol. 54, # 8, p. 918 - 920
[2] Tetrahedron Letters, 2009, vol. 50, # 31, p. 4459 - 4462
[3] European Journal of Medicinal Chemistry, 2013, vol. 65, p. 12 - 20
[4] Patent: WO2014/200872, 2014, A1. Location in patent: Page/Page column 73; 77
[5] Organic and Biomolecular Chemistry, 2011, vol. 9, # 5, p. 1591 - 1599 |
| | TRI-T-BUTYL 1 4 7 10-TETRAAZACYCLODODECA Preparation Products And Raw materials |
| Raw materials | tert-Butyl bromoacetate-->tert-Butyl chloroacetate-->Cyclen-->Acetonitrile-->Sodium bicarbonate | | Preparation Products | DOTA-mono-NHS tris(t-Bu ester)-->1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid, 1-(2,5-dioxo-1-pyrrolidinyl) ester-->1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid, tris(1,1-diMethylethyl) phenylMethyl ester |
|