N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide manufacturers
|
| | N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide Basic information |
| Product Name: | N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide | | Synonyms: | Cabotinib Impurity 7;1-(4-fluorophenyl)-N1-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide;N-(4-fluorophenyl)-N-(4-hydroxyphenyl)-1,1-Cyclopropanedicarboxamide;N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide;Cyclopropane-1,1-dicarboxylic acid N-(4-fluorophenyl)amide N'-(4-hydroxyphenyl)amide;cyclopropane-1,1-dicarboxylic acid (4-fluoro-phenyl)-amide (4-hydroxy-phenyl)-amide;1-N'-(4-fluorophenyl)-1-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide;1,1-Cyclopropanedicarboxamide, N-(4-fluorophenyl)-N'-(4-hydroxyphenyl)- | | CAS: | 849217-60-3 | | MF: | C17H15FN2O3 | | MW: | 314.31 | | EINECS: | | | Product Categories: | | | Mol File: | 849217-60-3.mol | 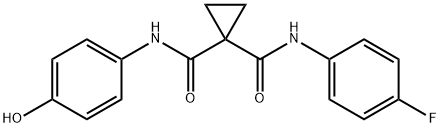 |
| | N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide Chemical Properties |
| Boiling point | 639.7±50.0 °C(Predicted) | | density | 1.486±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | pka | 10.11±0.26(Predicted) | | Appearance | Off-white to light brown Solid | | InChI | InChI=1S/C17H15FN2O3/c18-11-1-3-12(4-2-11)19-15(22)17(9-10-17)16(23)20-13-5-7-14(21)8-6-13/h1-8,21H,9-10H2,(H,19,22)(H,20,23) | | InChIKey | FSFMBVMXIPQPMJ-UHFFFAOYSA-N | | SMILES | C1(C(NC2=CC=C(O)C=C2)=O)(C(NC2=CC=C(F)C=C2)=O)CC1 |
| | N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide Usage And Synthesis |
| Uses | N-(4-Fluorophenyl)-N''-(4-hydroxyphenyl)-1,1-cyclopropanedicarboxamide can be used to treat kinase-dependenet disorders. | | Synthesis | GENERAL STEPS: To a solution of 4-aminophenol (2.93 g, 26.9 mmol) and 1-(4-fluorophenylcarbamoyl)cyclopropanecarboxylic acid (5.00 g, 22.4 mmol) in N,N-dimethylacetamide (DMA, 30 mL) was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 5.15 g, 26.9 mmol). The reaction mixture was stirred vigorously at room temperature until the reaction was complete (monitored by TLC, about 3 hours). Upon completion of the reaction, the reaction mixture was slowly poured into a flask containing saturated sodium bicarbonate (NaHCO3, 200 mL) aqueous solution under vigorous stirring. After continued stirring for 1 h, the resulting suspension was filtered. The solid product was collected, washed sequentially with water (50 mL) and chloroform (50 mL), and then dried under vacuum to afford N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide (6.22 g, 88% yield) as a white powder (HPLC purity >95%, and structure confirmed by 1H NMR). | | References | [1] Patent: WO2005/30140, 2005, A2. Location in patent: Page/Page column 205
[2] Patent: CN106632028, 2017, A. Location in patent: Paragraph 0079; 0084; 0085
[3] Patent: WO2005/30140, 2005, A2. Location in patent: Page/Page column 205
[4] European Journal of Medicinal Chemistry, 2017, vol. 140, p. 212 - 228 |
| | N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide Preparation Products And Raw materials |
|