4-CYANOBENZENE-1-SULFONAMIDE manufacturers
|
| | 4-CYANOBENZENE-1-SULFONAMIDE Basic information |
| Product Name: | 4-CYANOBENZENE-1-SULFONAMIDE | | Synonyms: | 4-Cyanobenzenesulphonamide 97%;4-Sulphamoylbenzonitrile;4-Sulphamoylbenzonitrile, 4-(Aminosulphonyl)benzonitrile;p-cyano-benzenesulfonamid;BUTTPARK 48\11-02;4-CYANOBENZENESULFONAMIDE;4-CYANOBENZENE-1-SULFONAMIDE;4-CYANOBENZENE-1-SULPHONAMIDE | | CAS: | 3119-02-6 | | MF: | C7H6N2O2S | | MW: | 182.2 | | EINECS: | 221-492-5 | | Product Categories: | SULFONAMIDE | | Mol File: | 3119-02-6.mol | 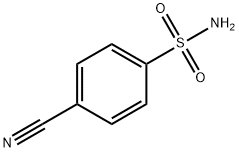 |
| | 4-CYANOBENZENE-1-SULFONAMIDE Chemical Properties |
| Melting point | 166 °C | | Boiling point | 400.7±47.0 °C(Predicted) | | density | 1.3827 (rough estimate) | | refractive index | 1.6000 (estimate) | | RTECS | DB1582000 | | storage temp. | Sealed in dry,Room Temperature | | pka | 9.57±0.10(Predicted) | | form | solid | | color | Off-white | | Water Solubility | 1.111g/L(15 ºC) | | CAS DataBase Reference | 3119-02-6(CAS DataBase Reference) |
| | 4-CYANOBENZENE-1-SULFONAMIDE Usage And Synthesis |
| Uses | 4-Cyanobenzene-1-sulfonamide is a carbonic anhydrase inhibitor. | | Synthesis | General procedure for the synthesis of p-cyanobenzenesulfonamide from 4-cyanobenzenesulfonyl chloride: 4-cyanobenzenesulfonyl chloride (10 mmol) was dissolved in anhydrous tetrahydrofuran (40 mL). A 30% aqueous ammonium hydroxide solution (3 mL) was slowly added to the reaction mixture at 0 °C, followed by stirring the reaction for 1 h at room temperature. After completion of the reaction, the reaction mixture was extracted with ethyl acetate and the organic phase was dried with anhydrous sodium sulfate. After concentration under reduced pressure, the white solid product p-cyanobenzenesulfonamide (1.6 g, 88% yield) was obtained by grinding with ether. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6) and 13C NMR (100 MHz, DMSO-d6): 1H NMR δ 8.11 (2H, d, J = 8.58 Hz), 8.02 (2H, d, J = 8.62 Hz), 7.69 (2H, bs, NH2, exchange with D2O); 13C NMR δ 148.9, 134.2, 127.4, 118.8, 115.3. | | References | [1] European Journal of Medicinal Chemistry, 2018, vol. 157, p. 1214 - 1222
[2] Zhurnal Obshchei Khimii, 1948, vol. 18, p. 110,112
[3] Chem.Abstr., 1948, p. 4976
[4] American Chemical Journal, 1896, vol. 18, p. 163,165
[5] Bioorganic and Medicinal Chemistry, 2017, vol. 25, # 16, p. 4397 - 4406 |
| | 4-CYANOBENZENE-1-SULFONAMIDE Preparation Products And Raw materials |
|