Product Number: A047057
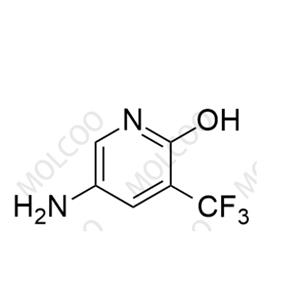
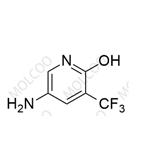
English Name: Apalutamide Impurity 57
English Alias: 5-amino-3-(trifluoromethyl)pyridin-2-ol
CAS Number: 1373232-58-6
Molecular Formula: C6H5F3N2O
Molecular Weight: 178.11
Advantages: Apalutamide Impurity 57 is synthesized through advanced and refined processes, combined with strict multi-level purification techniques. This results in an exceptionally high-purity product with outstanding batch-to-batch consistency. The chemical structure of the product is accurately verified using sophisticated analytical methods such as high-resolution mass spectrometry (HRMS) and nuclear magnetic resonance (NMR). With impurity residues far below the industry standard, it serves as a highly reliable reference substance for drug research, development, and quality control, effectively guaranteeing the accuracy and repeatability of experimental data.
Applications: Primarily applied to the impurity analysis, quality control, and safety evaluation of Apalutamide drugs. During the drug research and development phase, it is used to determine the content of this impurity in drugs, and to assess its potential impacts on drug efficacy and toxicity. In the production process, as a crucial reference standard, it enables the precise detection of impurity levels in pharmaceutical products, ensuring compliance with international regulations such as those set by the ICH. Additionally, it is valuable for studies on drug stability and the analysis of degradation pathways.
Background Description: Apalutamide, an important drug for the treatment of prostate cancer, has impurity control as a core component of its pharmaceutical quality system. As regulatory authorities increasingly tighten the limits on drug impurities, research on Apalutamide Impurity 57 has become pivotal in ensuring drug safety. The presence of this impurity may potentially influence the purity and therapeutic effect of the drug. Therefore, its accurate characterization and strict control are of great clinical significance.
Research Status: Currently, research on Apalutamide Impurity 57 mainly focuses on the development of highly sensitive detection methods and the exploration of its generation mechanisms. Researchers utilize advanced techniques such as ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) to achieve trace analysis of this impurity. At the same time, efforts are being made to optimize the synthesis routes to reduce its formation during the drug production process. Moreover, studies on the impact of this impurity on drug stability and biological activity are gradually progressing, providing a theoretical basis for formulating more scientific impurity control strategies.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!










 China
China