
Product Details
| Product Name: Chloroprocaine Impurity | Min. Order: 10mg |
| Purity: 99%+ HPLC | Supply Ability: 1000 |
| Release date: 2025/07/31 |
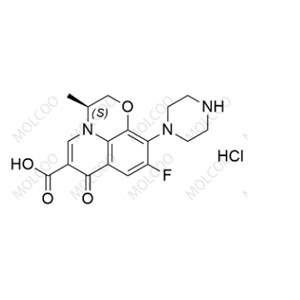
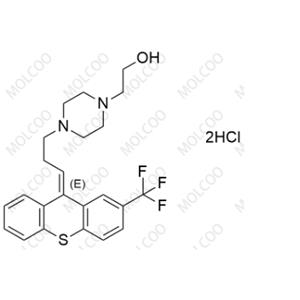
Chloroprocaine Impurity

WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!
Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1KG |
VIP2Y
|
Shaanxi Xianhe Biotech Co., Ltd
|
2025-04-21 | |
| $1980.00/50mg |
VIP2Y
|
TargetMol Chemicals Inc.
|
2025-08-21 | |
| $38.00/5mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-09-18 |
INQUIRY










 China
China