Dienogest Impurity
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: D046016
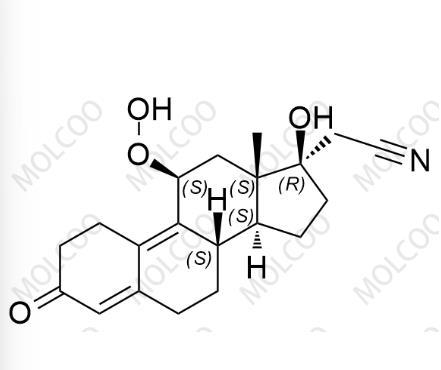
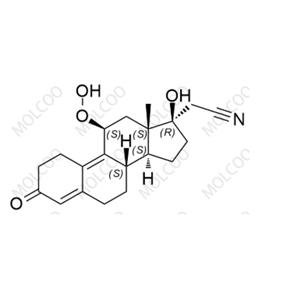
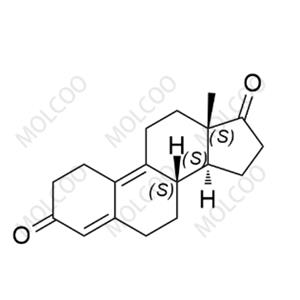
English Name: Dienogest Impurity 16
English Alias: 2-((8S,11S,13S,14S,17R)-11-hydroperoxy-17-hydroxy-13-methyl-3-oxo-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)acetonitrile
CAS Number: 106111-43-7
Molecular Formula: C₂₀H₂₅NO₄
Molecular Weight: 343.42
Product Advantages: Dienogest Impurity 16 has high purity and good chemical stability. Its structure is confirmed by precise spectroscopic analysis (such as nuclear magnetic resonance and mass spectrometry), and it remains stable and uniform under different experimental environments and storage conditions. As a reference substance, its precision ensures the accuracy and repeatability of Dienogest impurity detection results, providing a reliable basis for pharmaceutical quality research and quality control, and facilitating quality control in drug research and production processes.
Application Fields:
Quality Control: As an impurity reference standard, it is used to establish and validate the detection methods for impurities in Dienogest bulk drugs and formulations, ensuring that the sensitivity and specificity of the detection methods meet pharmaceutical quality standard requirements and guaranteeing drug quality and safety.
Process Optimization: During the production of Dienogest, by monitoring the content of this impurity and analyzing the stages of its generation, it assists in optimizing the synthesis process, reducing impurity formation, and improving product quality.
Stability Studies: In drug stability tests, it analyzes the changes of this impurity under different storage conditions (such as temperature, humidity, light), providing data support for determining the shelf life and storage conditions of drugs.
Background Description: Dienogest is a progestogen drug used for the treatment of endometriosis and other diseases. In the process of its research, development, production, and quality control, impurity research is a key link to ensure drug safety and effectiveness. The presence of impurities may affect the drug's efficacy and even cause potential toxic and side effects. As a related impurity of Dienogest, in-depth research on Dienogest Impurity 16 helps to comprehensively evaluate the quality of Dienogest drugs and ensure the safety and effectiveness of clinical medication.
Research Status: Currently, research on Dienogest Impurity 16 mainly focuses on detection technology and impurity generation mechanisms. In terms of detection technology, advanced methods such as Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry are constantly being explored to achieve precise detection of trace impurities. In the study of impurity generation mechanisms, the formation causes and influencing factors are analyzed by simulating drug synthesis reactions and storage environments, providing a theoretical basis for controlling impurities from the source. In addition, research on the impact of this impurity on drug efficacy and safety is also gradually being carried out, aiming to improve the comprehensive understanding of the quality of Dienogest drugs.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!










 China
China