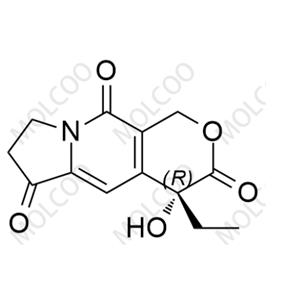
Product Details
| Product Name: Exatecan Impurity | CAS No.: 110351-91-2 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
Exatecan Impurity

Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-06 | |
| $0.00/1KG |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2025-09-11 | |
| $30.00/10mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-11-03 |
INQUIRY








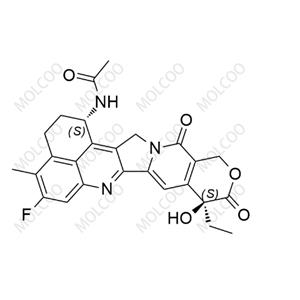
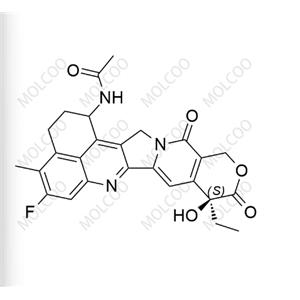


 China
China