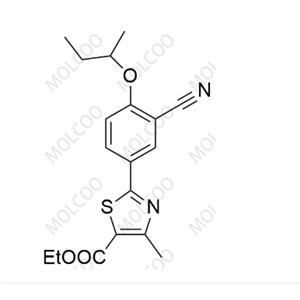
Product Details
| Product Name: Febuxostat Impurity | CAS No.: 2375033-35-3 |
| Min. Order: 10mg | Purity: 98 |
| Supply Ability: 1000000000 | Release date: 2025/07/31 |

Our products are mainly drug impurity reference products, drug standards, analytical chemistry, standard substances, organic chemical synthesis, chemical custom synthesis. For impurity localization, quantification, and quality control in drug development processes. Our laboratory area is more than 5000 square meters, we have more than 20 analytical instruments such as HPLC and MS/LC-MS/GC-MS, we also have TGA, IR and other testing instruments. We have the most advanced SFC preparation separation equipment.
All of our products include a complete set of structural confirmation maps. Such as COA, H-NMR,MASS,HPLC,UV,IR。The default HPLC purity of our products is not less than 95%. Particularly unstable products have a purity of no less than 90% and will be communicated to the customer in advance. we can also provide TGA,Q-NMR,C-NMR and so on。

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/25Kg/Drum |
VIP5Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2021-09-09 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-07-10 | |
| $0.00/25Kg/Bag |
VIP5Y
|
Sinoway Industrial co., ltd.
|
2022-08-08 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-07-04 |







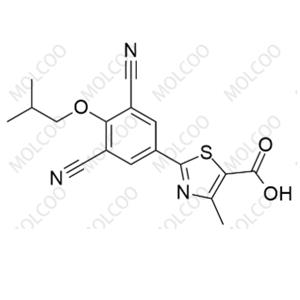
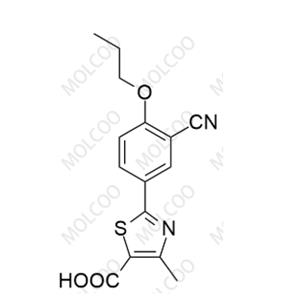
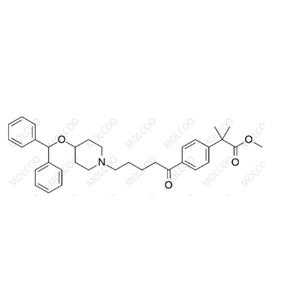

 China
China