
Product Details
| Product Name: Isavuconazole Impurity 4(Dihydrochloride) | CAS No.: 2732924-99-9 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C10H15N3O2.2HCl |
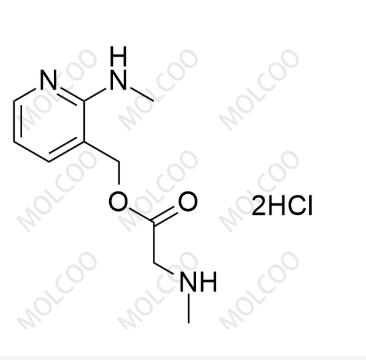
Isavuconazole Impurity 4(Dihydrochloride);2732924-99-9
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
- .
Product Information
Product Number: I004004A
English Name: Isavuconazole Impurity 4(Dihydrochloride)
English Alias: (2-(methylamino)pyridin-3-yl)methyl 2-(methylamino)acetate dihydrochloride
CAS Number: 2732924-99-9
Molecular Formula: C₁₀H₁₅N₃O₂·2HCl
Molecular Weight: 282.16(209.24 + 2×36.46)
Advantages
Well-defined structure and stable salt form: Clear pyridine methyl ester and dihydrochloride structure, containing methylamino and ester groups, which can be used to analyze the by-product formation mechanism of pyridine ring amination and esterification reactions in isavuconazole synthesis, optimizing processes to reduce impurity generation;
Multifunctional standard substance: Composite structure with pyridine ring, amino group, ester group and chloride ion, providing an accurate standard for detection methods such as HPLC and LC-MS to improve the separation and quantification accuracy of nitrogen-containing heterocyclic impurities;
Water solubility advantage: The dihydrochloride form significantly enhances the water solubility of the compound, facilitating accurate detection in formulation stability studies and physiological environment simulation experiments, and improving the applicability of detection methods.
Applications
Drug Development: Used as an impurity reference standard to identify and quantify Isavuconazole Impurity 4(Dihydrochloride) in isavuconazole and its formulations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC, LC-MS), ensuring impurity content meets pharmacopoeia and regulatory requirements during production;
Process Optimization: Reducing by-product generation by monitoring the generation amount of this impurity and optimizing the catalyst (such as potassium carbonate) for pyridine ring methylation and esterification temperature.
Background Description
Research Status
Detection method optimization: Establishing trace detection methods (detection limits reach ppb level) using UPLC-MS/MS technology, enhancing mass spectrometry response by leveraging the ionization characteristics of dihydrochloride;
Synthesis process improvement: Reducing the formation of over-aminated by-products of the pyridine ring by adjusting the solvent (e.g., methanol) and reaction time of the amination reaction;
Toxicological evaluation: Evaluating the potential interference of this impurity on the antifungal activity of isavuconazole through in vitro antifungal experiments to provide data for formulating reasonable impurity limit standards;
Stability studies: Analyzing the hydrolysis behavior (such as ester bond cleavage) of this impurity under high temperature and high humidity conditions to assess its impact on the storage stability of isavuconazole formulations
Product Information
Product Number: I004004A
English Name: Isavuconazole Impurity 4(Dihydrochloride)
English Alias: (2-(methylamino)pyridin-3-yl)methyl 2-(methylamino)acetate dihydrochloride
CAS Number: 2732924-99-9
Molecular Formula: C₁₀H₁₅N₃O₂·2HCl
Molecular Weight: 282.16(209.24 + 2×36.46)
Advantages
Well-defined structure and stable salt form: Clear pyridine methyl ester and dihydrochloride structure, containing methylamino and ester groups, which can be used to analyze the by-product formation mechanism of pyridine ring amination and esterification reactions in isavuconazole synthesis, optimizing processes to reduce impurity generation;
Multifunctional standard substance: Composite structure with pyridine ring, amino group, ester group and chloride ion, providing an accurate standard for detection methods such as HPLC and LC-MS to improve the separation and quantification accuracy of nitrogen-containing heterocyclic impurities;
Water solubility advantage: The dihydrochloride form significantly enhances the water solubility of the compound, facilitating accurate detection in formulation stability studies and physiological environment simulation experiments, and improving the applicability of detection methods.
Applications
Drug Development: Used as an impurity reference standard to identify and quantify Isavuconazole Impurity 4(Dihydrochloride) in isavuconazole and its formulations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC, LC-MS), ensuring impurity content meets pharmacopoeia and regulatory requirements during production;
Process Optimization: Reducing by-product generation by monitoring the generation amount of this impurity and optimizing the catalyst (such as potassium carbonate) for pyridine ring methylation and esterification temperature.
Background Description
Research Status
Detection method optimization: Establishing trace detection methods (detection limits reach ppb level) using UPLC-MS/MS technology, enhancing mass spectrometry response by leveraging the ionization characteristics of dihydrochloride;
Synthesis process improvement: Reducing the formation of over-aminated by-products of the pyridine ring by adjusting the solvent (e.g., methanol) and reaction time of the amination reaction;
Toxicological evaluation: Evaluating the potential interference of this impurity on the antifungal activity of isavuconazole through in vitro antifungal experiments to provide data for formulating reasonable impurity limit standards;
Stability studies: Analyzing the hydrolysis behavior (such as ester bond cleavage) of this impurity under high temperature and high humidity conditions to assess its impact on the storage stability of isavuconazole formulations
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-05-14 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-14 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-07-10 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-07-10 |









 China
China